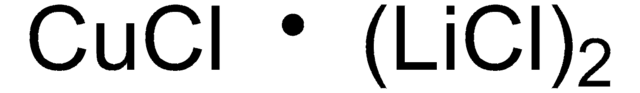900624
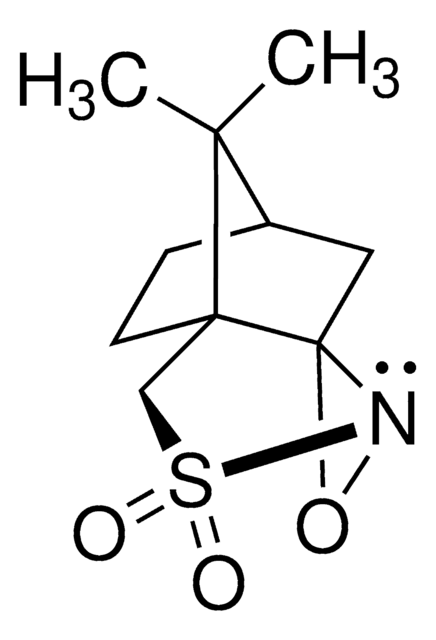
Di-t-butyl oxaziridine
≥95%
Synonym(s):
Kurti oxaziridine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H19NO
CAS Number:
Molecular Weight:
157.25
UNSPSC Code:
12352000
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
form
liquid
availability
available only in USA
refractive index
n/D 1.4453
density
0.90 g/mL
storage temp.
2-8°C
SMILES string
CC(C)(C)C1(NO1)C(C)(C)C
Application
This oxaziridine has been used in synthetic methods for amination, heteroatom transfer, and C-H functionalization. Recently, the lab of Laszlo Kurti demonstrated its application for direct electrophilic primary and secondary amination of arylmetals in the presence of Cu(I) salts -- void of precious metal catalysts, ligands, protecting groups, or directing groups.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Self-react. C
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flash Point(F)
143.6 °F
Flash Point(C)
62 °C
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of Alkoxylamines by Alkoxide Amination with 3,3'-Di-tert-butyloxaziridine.
Ingrid C. Choong et al.
The Journal of organic chemistry, 64(18), 6528-6529 (2001-10-25)
Zhe Zhou et al.
Journal of the American Chemical Society, 139(1), 115-118 (2016-12-23)
Herein we disclose a novel method for the facile transfer of primary (-NH2) and secondary amino groups (-NHR) to heteroaryl- as well as arylcuprates at low temperature without the need for precious metal catalysts, ligands, excess reagents, protecting and/or directing
Yan Xu et al.
Nature chemistry, 7(10), 829-834 (2015-09-24)
Site-selective C-H functionalization has emerged as an attractive tool for derivatizing complex synthetic intermediates, but its use for late-stage diversification is limited by the functional groups that can be introduced, especially at unactivated sp(3)-hybridized positions. To overcome this, we introduce
Hongyin Gao et al.
Nature chemistry, 9(7), 681-688 (2017-06-24)
Arylmetals are highly valuable carbon nucleophiles that are readily and inexpensively prepared from aryl halides or arenes and widely used on both laboratory and industrial scales to react directly with a wide range of electrophiles. Although C-C bond formation has
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service