735353
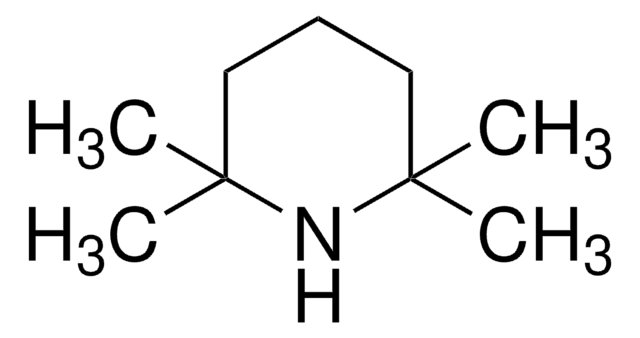
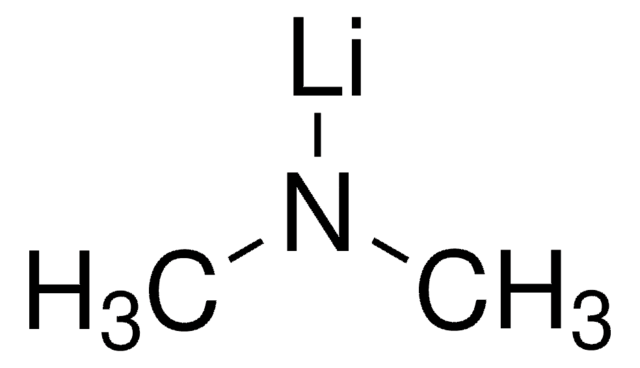
Lithium 2,2,6,6-tetramethylpiperidide
95%
Synonym(s):
2,2,6,6-Tetramethylpiperidine lithium salt (1:1), LTMP
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H18LiN
CAS Number:
Molecular Weight:
147.19
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
SMILES string
[Li]N1C(C)(C)CCCC1(C)C
InChI
1S/C9H18N.Li/c1-8(2)6-5-7-9(3,4)10-8;/h5-7H2,1-4H3;/q-1;+1
InChI key
ANYSGBYRTLOUPO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Lithium 2,2,6,6-tetramethylpiperidide is a strong base and can be used:
- For the synthesis of enamines from terminal epoxides through trans-α-lithiated epoxide as an intermediate.
- For ortholithiation of arenes such as 1,3-bis(trifluoromethyl)benzene, 4,4-dimethyl-2-phenyl-2-oxazoline, 1,4-bis(trifluoromethyl)benzene and 1,3-dimethoxybenzene.
- In combination with Lewis donor ligand, N,N,N′,N′-tetramethylethylenediamine (TMEDA) for deprotonative metalation of methoxy-substituted arenes.{21]
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B - Water-react 2
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Deprotonative metalation of methoxy-substituted arenes using lithium 2, 2, 6, 6-tetramethylpiperidide: Experimental and computational study.
Akimoto G, et al.
The Journal of Organic Chemistry, 83(21), 13498-13506 (2018)
Case for Lithium Tetramethylpiperidide-Mediated Ortholithiations: Reactivity and Mechanisms.
Mack KA and Collum DB
Journal of the American Chemical Society, 140(14), 4877-4883 (2018)
Martin-Louis Y Riu et al.
Science advances, 6(13), eaaz3168-eaaz3168 (2020-04-02)
This exploratory synthesis investigation was undertaken to determine the viability of replacing a single carbon vertex with another p-block element in a highly strained tetrahedrane molecule. Phosphorus was selected for this purpose because the stable molecular form of elemental phosphorus
Alkenes from terminal epoxides using lithium 2,2,6,6-tetramethylpiperidide and organolithiums or grignard reagents.
Hodgson DM, et al.
Journal of the American Chemical Society, 126(39), 12250-12251 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service