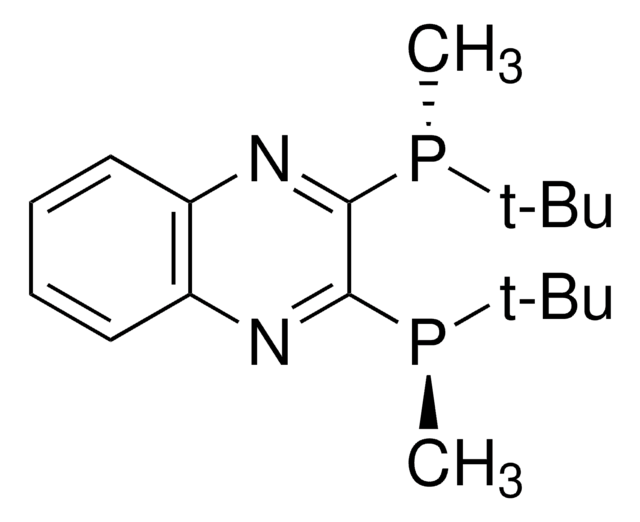
682144
(R)-(–)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane
96%
Synonym(s):
(R)-Phanephos
About This Item
Recommended Products
Quality Level
Assay
96%
form
solid
optical activity
[α]/D -34±4°, c = 1 in chloroform
mp
222-226 °C
functional group
phosphine
SMILES string
P(c7ccccc7)(c6ccccc6)c1c2ccc(c1)CCc3c(cc(cc3)CC2)P(c5ccccc5)c4ccccc4
InChI
1S/C40H34P2/c1-5-13-35(14-6-1)41(36-15-7-2-8-16-36)39-29-31-21-25-33(39)27-23-32-22-26-34(28-24-31)40(30-32)42(37-17-9-3-10-18-37)38-19-11-4-12-20-38/h1-22,25-26,29-30H,23-24,27-28H2
InChI key
GYZZZILPVUYAFJ-UHFFFAOYSA-N
Application
- Enantioselective reductive cyclization of 1,6-enynes via asymmetric hydrogenation in the presence of a rhodium catalyst to form alkylidene-substituted heterocycles.
- Asymmetric hydroboration of 3,3-disubstituted cyclopropenes to form 2,2-disubstituted cyclopropyl boronates.
- Asymmetric ring-opening reactions of azabenzonorbornadienes in the presence of zinc(II) triflate and palladium(II) acetate to form aminodihydronaphthalenes.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We present an article concerning P-Phos, PhanePhos and BoPhoz™ Ligands.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(S)-(+)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane 96%](/deepweb/assets/sigmaaldrich/product/structures/396/009/d814b698-3227-4aef-b415-cb5f5730aa13/640/d814b698-3227-4aef-b415-cb5f5730aa13.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![1,1′-Bis[(2R,5R)-2,5-diethylphospholano]ferrocene](/deepweb/assets/sigmaaldrich/product/structures/163/400/0628df6f-e028-4669-8def-26e6eb4f4743/640/0628df6f-e028-4669-8def-26e6eb4f4743.png)
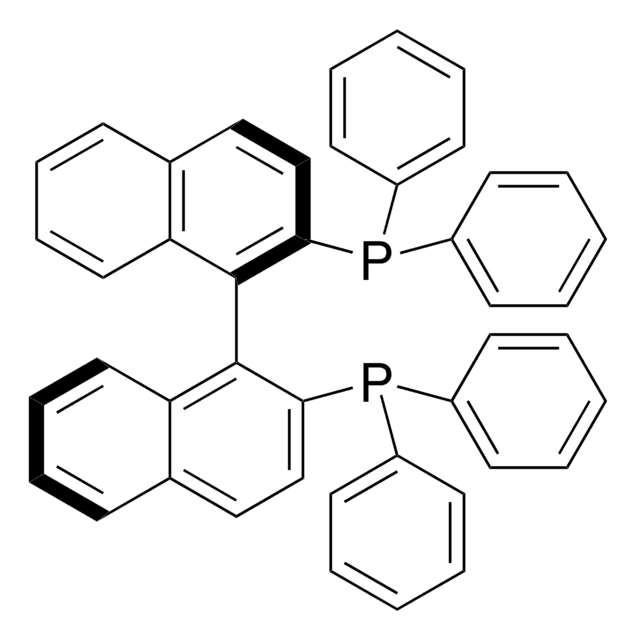
![[2.2]Paracyclophane 97%](/deepweb/assets/sigmaaldrich/product/structures/165/940/d2dda3d5-1fe9-4c87-9a85-009490e67661/640/d2dda3d5-1fe9-4c87-9a85-009490e67661.png)

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)