559938
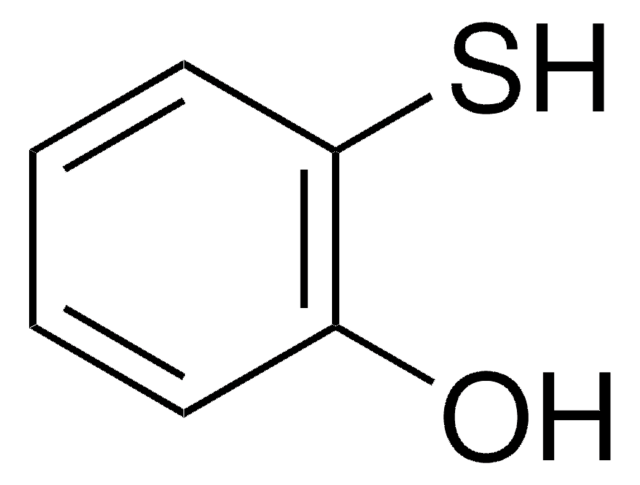
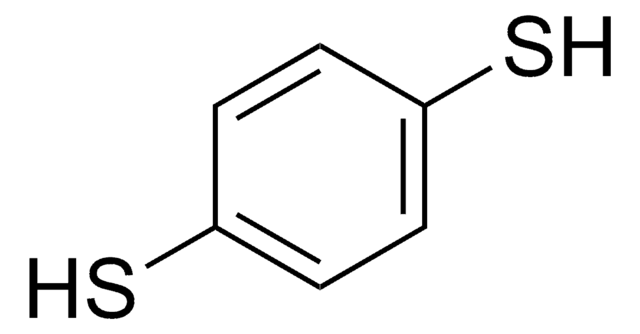
4-Mercaptophenol
97%
Synonym(s):
4-Hydroxythiophenol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HSC6H4OH
CAS Number:
Molecular Weight:
126.18
Beilstein:
2039306
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
bp
149-150 °C/25 mmHg (lit.)
mp
33-35 °C (lit.)
SMILES string
Oc1ccc(S)cc1
InChI
1S/C6H6OS/c7-5-1-3-6(8)4-2-5/h1-4,7-8H
InChI key
BXAVKNRWVKUTLY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Mercaptophenol (MPH) has been used to study the adsorption of MPH on silver coated polystyrene nanospheres by time-dependent surface-enhanced Raman scattering (SERS) spectroscopy.
4-Mercaptophenol may be used in the preparation of silylated monomer, which was employed for the synthesis of hyperbranched poly(ester-imide). 4-Mercaptophenol (H-4MP) reacts with metal tert-butoxides ([M(OBut)4]) to yield the following Group 4 phenoxy-thiols:
4-Mercaptophenol may be used in the preparation of silylated monomer, which was employed for the synthesis of hyperbranched poly(ester-imide). 4-Mercaptophenol (H-4MP) reacts with metal tert-butoxides ([M(OBut)4]) to yield the following Group 4 phenoxy-thiols:
- [(HOBut)(4MP)3M(μ-4MP)]2, where M = Ti, Zr, Hf
- [(py)2M(4MP)], where M = Ti, Zr; py = pyridine
- [(py)(4MP)3Hf(μ-4MP)]2
- poly(ethersulfide)s via silylation followed by polycondensation with 2,6-dichloropyridine or 3,6-dichloropyridazine
- 2,6-di-tertiarybutyl-4-mercaptophenol via Friedel-Craft′s alkylation with tert-butyl chloride in presence of a lewis acid
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and characterization of a series of Group 4 phenoxy-thiol derivatives.
Boyle TJ, et al.
Polyhedron, 110, 1-13 (2016)
Nouf N Mahmoud et al.
Biomaterials science, 8(6), 1669-1682 (2020-01-28)
The blood brain barrier (BBB) is a very selective barrier that protects the brain and the central nervous system (CNS) from the entry of harmful substances and helps regulate the exchange of different molecules and nutrients from and into the
New polymer synthesis 99. Hyperbranched poly (ester-imide) s derived from 4, 5-dichlorophthalic acid.
Kricheldorf HR, et al.
High Performance Polymers, 10(3), 217-229 (1998)
Optimization of Ag-Coated Polystyrene Nanosphere Substrates for Quantitative Surface-Enhanced Raman Spectroscopy Analysis.
Ingram WM, et al.
The Journal of Physical Chemistry C, 119(49), 27639-27648 (2015)
Sriram.D and Yogeeswari.P
Medicinal Chemistry null
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service