All Photos(1)
About This Item
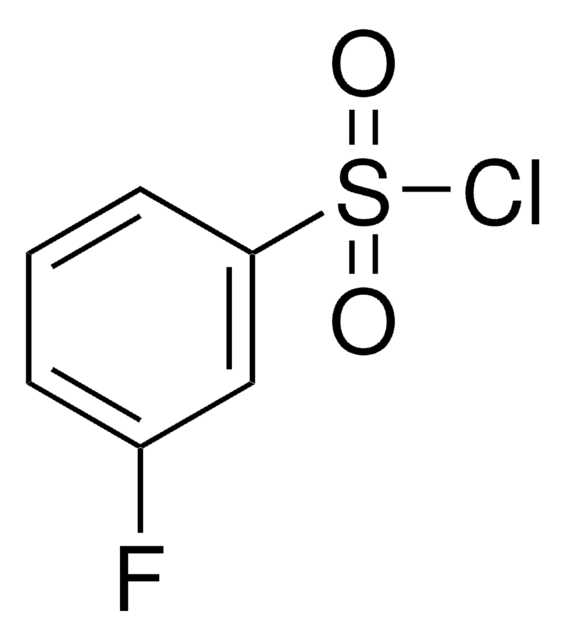
Linear Formula:
FC6H4SO2Cl
CAS Number:
Molecular Weight:
194.61
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.537 (lit.)
bp
246-247 °C (lit.)
mp
27-30 °C (lit.)
density
1.47 g/mL at 25 °C (lit.)
functional group
fluoro
SMILES string
Fc1ccccc1S(Cl)(=O)=O
InChI
1S/C6H4ClFO2S/c7-11(9,10)6-4-2-1-3-5(6)8/h1-4H
InChI key
ZSZKAQCISWFDCQ-UHFFFAOYSA-N
General description
2-Fluorobenzenesulfonyl chloride, also known as o-fluorobenzenesulfonyl chloride, is a flurinated arylsulfonyl chloride. It can be prepared from o-benzenedisulfonyl fluoride.
Application
2-Fluorobenzenesulfonyl chloride may be used in the preparation of the following furan derivatives:
It may also be used to prepare:
- 2-(2-fluorophenyl)benzofuran
- 2-butyl-5-(2-fluorophenyl)furan
- 2-(2-fluorophenyl)-3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran
It may also be used to prepare:
- 2-fluorobenzenesulfonamide
- methyl 2-{[(2-fluorophenyl)sulfonyl]amino}-5,6,7,8-tetrahydro-1-naphthalenecarboxylate
- potassium fluorobenzene-2-sulfonate
- 1-(2-bromobenzyl)-2-(2-fluorophenyl)pyrrole
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Discovery and optimization of anthranilic acid sulfonamides as inhibitors of methionine aminopeptidase-2: a structural basis for the reduction of albumin binding.
Sheppard GS, et al.
Journal of Medicinal Chemistry, 49(13), 3832-3849 (2006)
Water-Soluble Phosphines. 6.1 Tailor-Made Syntheses of Chiral Secondary and Tertiary Phosphines with Sulfonated Aromatic Substituents: Structural and Quantum Chemical Studies.
Bitterer F, et al.
Inorganic Chemistry, 35(14), 4103-4113 (1996)
Regiocontroled Palladium?Catalysed Direct Arylation at Carbon C2 of Benzofurans using Benzenesulfonyl Chlorides as the Coupling Partners.
Loukotova L, et al.
ChemCatChem, 6(5), 1303-1309 (2014)
Potassium fluoride catalyzed fluorodesulfonylations of aryl sulfonyl fluorides.
Van der Puy M.
The Journal of Organic Chemistry, 53(18), 4398-4401 (1988)
Benzenesulfonyl Chlorides: Alternative Coupling Partners for Regiocontrolled Palladium-Catalyzed Direct Desulfitative 5-Arylation of Furans.
Beladhria A, et al.
Synthesis, 46(18), 2515-2523 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service