All Photos(1)
About This Item
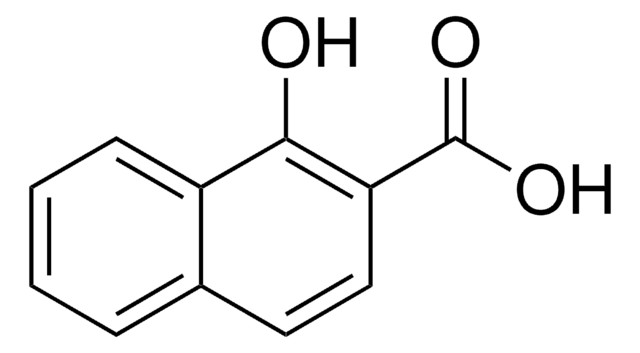
Linear Formula:
CH3C10H6CO2H
CAS Number:
Molecular Weight:
186.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
97%
mp
179-181 °C (lit.)
SMILES string
Cc1ccc(C(O)=O)c2ccccc12
InChI
1S/C12H10O2/c1-8-6-7-11(12(13)14)10-5-3-2-4-9(8)10/h2-7H,1H3,(H,13,14)
InChI key
SIVYRLBDAPKADZ-UHFFFAOYSA-N
Related Categories
General description
4-Methyl-1-naphthoic acid is a naphthoic acid derivative
Application
4-Methyl-1-naphthoic acid may be used to synthesize:
- 2-methyl-1-propyl-3-(4-methyl-1-naphthoyl)indole, JWH-148
- 4-methyl-1-naphthylcarbinol
- 1,4-dioxan-2-yl 4-methyl-1-naphthoate
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jincan Zhao et al.
The Journal of organic chemistry, 79(9), 3847-3855 (2014-04-15)
An iron-catalyzed oxidative esterification reaction between unactivated C(sp(3))-H bonds from symmetric and asymmetric ethers and carboxylic acids using di-tert-butyl peroxide (DTBP) as the oxidant via a cross dehydrogenative coupling (CDC) reaction was established, which tolerates a wide range of cyclic
John W Huffman et al.
Bioorganic & medicinal chemistry, 13(1), 89-112 (2004-12-08)
In an effort to improve indole-based CB(2) cannabinoid receptor ligands and also to develop SAR for both the CB(1) and CB(2) receptors, 47 indole derivatives were prepared and their CB(1) and CB(2) receptor affinities were determined. The indole derivatives include
Preparation of a series of substituted fluoromethylnaphthalenes.
Dixon EA, et al.
Canadian Journal of Chemistry, 59(17), 2629-2641 (1981)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service