All Photos(1)
About This Item
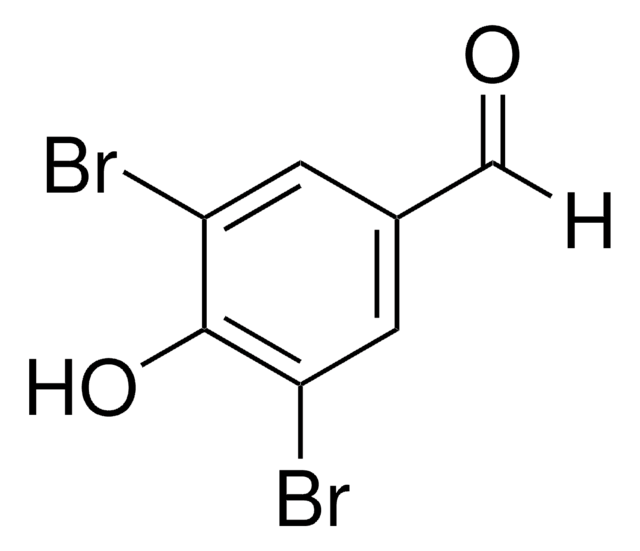
Linear Formula:
BrC6H3(OH)CHO
CAS Number:
Molecular Weight:
201.02
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
130-135 °C (lit.)
SMILES string
Oc1ccc(C=O)cc1Br
InChI
1S/C7H5BrO2/c8-6-3-5(4-9)1-2-7(6)10/h1-4,10H
InChI key
UOTMHAOCAJROQF-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Formation of 3-bromo-4-hydroxybenzaldehyde from L-tyrosine in cell-free homogenates of Odonthalia floccosa (Rhodophyceae): a proposed biosynthetic pathway for brominated phenols.
Manley SL and Chapman DJ.
Febs Letters, 93(1), 97-101 (1978)
S L Manley et al.
Plant physiology, 64(6), 1032-1038 (1979-12-01)
The biosynthesis of 4-hydroxybenzaldehyde and 3-bromo-4-hydroxybenzaldehyde from l-[U-(14)C]tyrosine has been demonstrated in chloroplast-containing fractions obtained by differential and isopycnic centrifugation from the marine red alga Odonthalia floccosa. Surfactant and high speed centrifugation studies indicate that the biosynthetic pathway involves a
C Flodin et al.
Phytochemistry, 53(1), 77-80 (2000-02-03)
The red marine alga Polysiphonia sphaerocarpa was extracted by a simultaneous steam distillation-solvent extraction technique and several brominated compounds were identified by gas chromatography-mass spectrometry. The compounds detected were 2,4-dibromoanisole, 2,4,6-tribromoanisole, 3-bromocresol, 3,5-dibromocresol, 3-bromo-4-hydroxybenzaldehyde, 3,5-dibromo-4-hydroxybenzaldehyde, 2-bromophenol, 4-bromophenol, 2,4-dibromophenol, 2,6-dibromophenol and
Hard acid and soft nucleophile system. New efficient method for removal of benzyl protecting group.
Fuji K, et al.
The Journal of Organic Chemistry, 44(10), 1661-1664 (1979)
Eun-Hye Kim et al.
Journal of materials chemistry. B, 8(44), 10162-10171 (2020-10-24)
Monoclonal antibodies have been developed as anticancer agents to block immune checkpoint pathways associated with programmed cell death 1 (PD-1) and its ligand PD-L1. However, the high cost of antibodies has encouraged researchers to develop other inhibitor types. Here, biphenyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)