481505
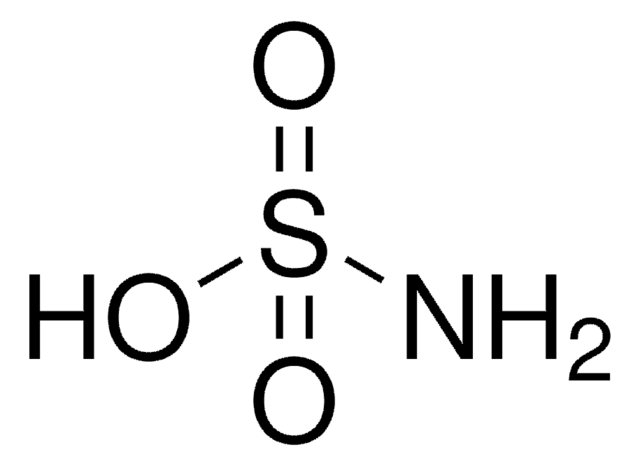
Sulfamic acid
99.999% trace metals basis
Synonym(s):
Amidosulfonic acid
About This Item
Recommended Products
Assay
99.999% trace metals basis
form
crystals
mp
215-225 °C (dec.) (lit.)
density
2.151 g/cm3 at 25 °C
anion traces
chloride (Cl-): ≤0.001%
sulfate (SO42-): ≤0.05%
cation traces
Fe: ≤5 ppm
heavy metals (as Pb): ≤0.001%
SMILES string
NS(O)(=O)=O
InChI
1S/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4)
InChI key
IIACRCGMVDHOTQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Sulfamic acid is an amino acid with sulfur and is mildly acidic. It acts as a green catalyst in the esterification reaction.
Application
- Friedlander quinoline synthesis.
- Liquid Beckmann rearrangement for the synthesis of amides from ketoximes.
- The preparation of α-aminophosphonates via a three-component reaction between aldehydes, amines, and diethyl phosphite.
Features and Benefits
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service