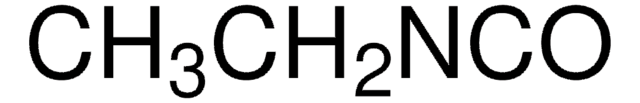All Photos(1)
About This Item
Linear Formula:
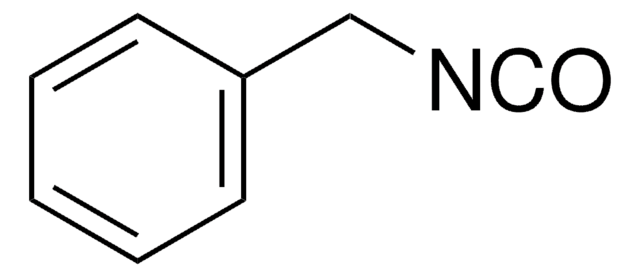
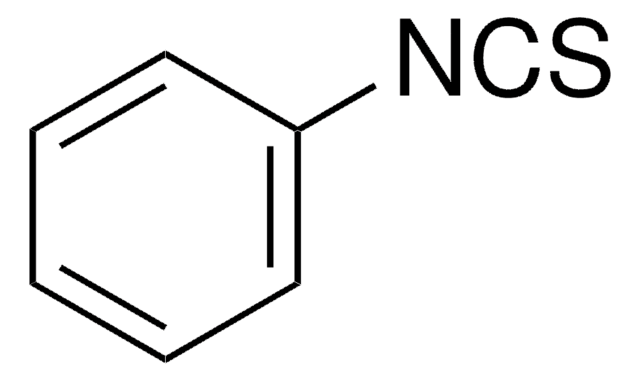
C6H5CH2CH2NCO
CAS Number:
Molecular Weight:
147.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.522 (lit.)
bp
210 °C (lit.)
density
1.063 g/mL at 25 °C (lit.)
SMILES string
O=C=NCCc1ccccc1
InChI
1S/C9H9NO/c11-8-10-7-6-9-4-2-1-3-5-9/h1-5H,6-7H2
InChI key
HACRKYQRZABURO-UHFFFAOYSA-N
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 2 - Resp. Sens. 1 - Skin Corr. 1A - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
212.0 °F - closed cup
Flash Point(C)
100 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Andreas Habel et al.
Journal of chromatography. A, 1165(1-2), 182-190 (2007-08-19)
1-phenylethyl isocyanate (1-PEIC), a chiral derivatisation reagent for the resolution of secondary alcohols is a powerful tool to determine the configuration and enantiomeric excess of medium- to long-chain secondary alcohols by capillary gas chromatography. The separation of 1-phenylethylcarbamates (1-PECs) of
J Gal et al.
Drug metabolism and disposition: the biological fate of chemicals, 9(6), 557-560 (1981-11-01)
Chiral secondary alcohols were treated with (S)-(-)-1-phenylethyl isocyanate. For each racemic alcohol, the resulting diastereomeric urethane derivatives were resolved on flexible fused-silica capillary GLC columns with retention times of 15 min or less. Derivatization of individual enantiomers showed that the
Silica gel high-performance liquid chromatography for the simultaneous determination of propranolol and 4-hydroxypropranolol enantiomers after chiral derivatization.
M J Wilson et al.
Journal of chromatography, 310(2), 424-430 (1984-10-12)
E Albano et al.
Hepatology (Baltimore, Md.), 23(1), 155-163 (1996-01-01)
We have previously shown that the treatment with diallyl sulfide (DAS) and phenylethyl isothiocyanate (PIC) of rats receiving ethanol in the alcohol tube-feeding model effectively suppressed the induction of cytochrome P4502E1 (CYP2E1) by ethanol. Here we report that rat treatment
A Adesida et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 34(4), 385-392 (1996-04-01)
Mercapturic acid pathway metabolites of phenylethyl isothiocyanate inhibited the growth of human leukaemia 60 (HL60) cells in vitro. The adduct with L-cysteine, S-(N-phenylethylthiocarbamoyl)cysteine, was the most potent with strong antileukaemic activity: the median growth inhibitory concentration (GC50) value was 336
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service