All Photos(1)
About This Item
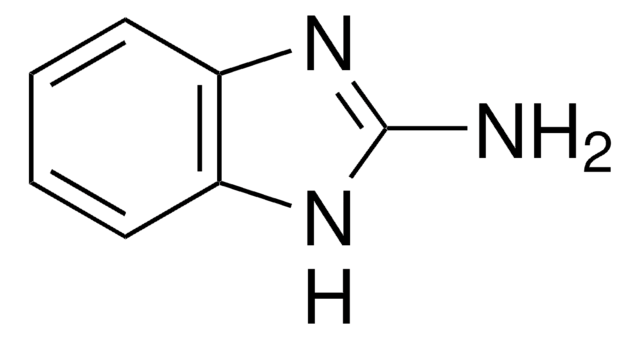
Empirical Formula (Hill Notation):
C3H4N2O
CAS Number:
Molecular Weight:
84.08
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.511 (lit.)
bp
226-228 °C (lit.)
density
1.138 g/mL at 25 °C (lit.)
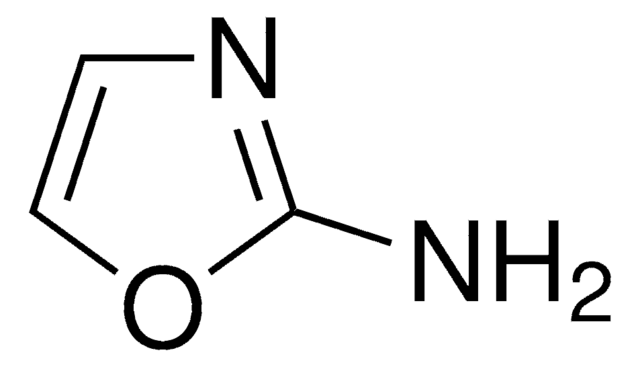
SMILES string
Nc1ccon1
InChI
1S/C3H4N2O/c4-3-1-2-6-5-3/h1-2H,(H2,4,5)
InChI key
RHFWLPWDOYJEAL-UHFFFAOYSA-N
General description
3-Aminoisoxazole (isoxazol-3-amine) is a 3-substituted isoxazole derivative. It is a structural isomer of 5-aminoisoxazole.
Application
3-Aminoisoxazole (isoxazol-3-amine) may be used in the following studies:
- As a reagent in the synthesis of N-(4-(N-isoxazol-3-ylsulfamoyl)phenyl)acetamide.
- As a starting material in the synthesis of N-(isoxazol-3-yl)-N′-(carbomethoxy)thiourea.
- As a starting material in the synthesis of (Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-methoxy-iminoacetic acid, a side-chain of the fourth generation of cephem antibiotics.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F
Flash Point(C)
113 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 3-and 5-amino-5-(3)-(pyrrol-2-yl) isoxazoles.
Lyubov'N S, et al.
Tetrahedron, 61(20), 4841-4849 (2005)
Martin J. Walsh et al.
Probe Reports from the NIH Molecular Libraries Program, 2011 Oct 18 (Updated 2013 Feb 25) (2013-09-13)
The protist
Tomasz Glinka et al.
Bioorganic & medicinal chemistry, 11(4), 591-600 (2003-01-23)
SAR studies in a series of related 3-(heteroarylthio)cephems determined that a relatively high chemical reactivity of the beta-lactam ring, modulated by electronic effects of substituents at C-3 and C-7, is necessary to achieve high in vitro activity against methicillin-resistant Staphylococcus
Kuniaki Tatsuta
Proceedings of the Japan Academy. Series B, Physical and biological sciences, 84(4), 87-106 (2008-10-23)
The first total synthesis and development of a variety of bioactive natural products have been accomplished by using carbohydrates as a chiral source. In addition, practically useful intermediates have been created, analogs of natural products have been prepared, their structure-activity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service