All Photos(3)
About This Item
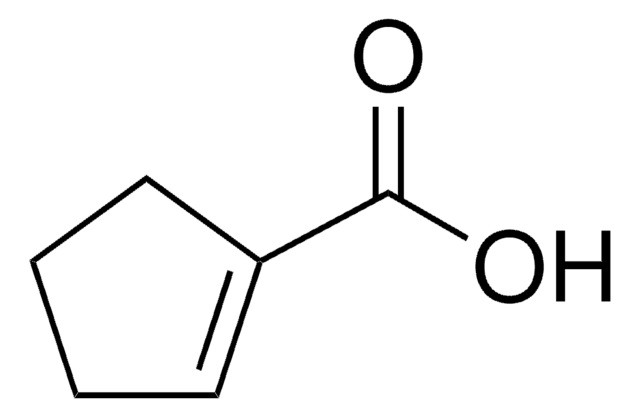
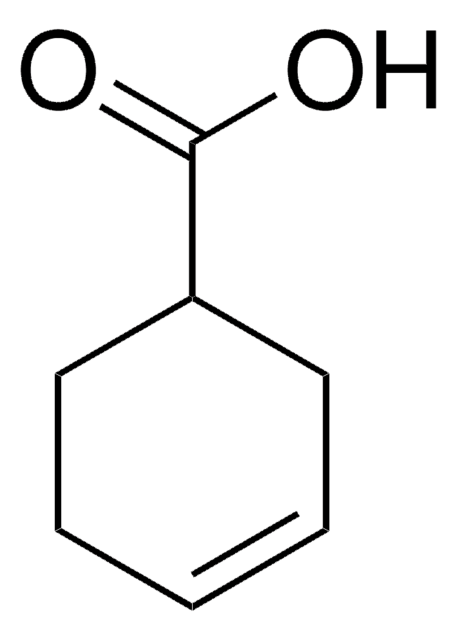
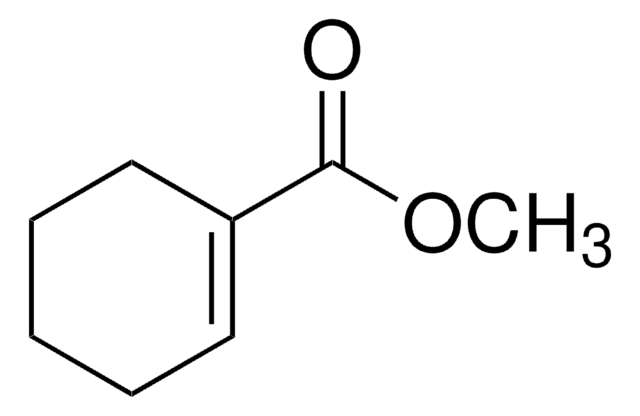
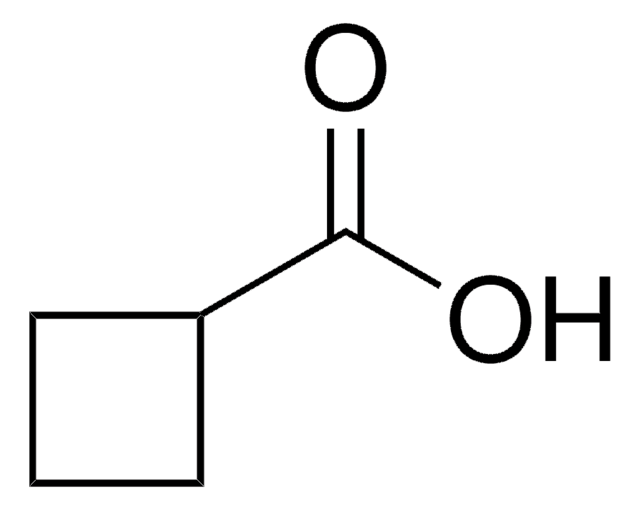
Linear Formula:
C6H9CO2H
CAS Number:
Molecular Weight:
126.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
133-135 °C/14 mmHg (lit.)
mp
35-39 °C (lit.)
density
1.101 g/mL at 25 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)C1=CCCCC1
InChI
1S/C7H10O2/c8-7(9)6-4-2-1-3-5-6/h4H,1-3,5H2,(H,8,9)
InChI key
NMEZJSDUZQOPFE-UHFFFAOYSA-N
Related Categories
General description
1-Cyclohexene-1-carboxylic acid was identified as intermediate during the anaerobic decomposition of benzoic acid by a methanogenic consortium.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C L Keith et al.
Archives of microbiology, 118(2), 173-176 (1978-08-01)
A possible pathway for the anaerobic utilization of benzoic acid by a methanogenic consortium is suggested. Cyclohexane carboxylic acid and 1-cyclohexene-1-carboxylic acid have been identified as intermediates before ring rupture. Suprisingly, 3-cyclohexene-1-carboxylic acid interferes with utilization of other cyclic acids.
Mariana G de Oliveira et al.
American journal of physiology. Renal physiology, 315(3), F460-F468 (2018-05-03)
Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) is a chronic inflammatory disease without consistently effective treatment. We investigate the role of toll-like receptor 4 (TLR4) on voiding dysfunction and inflammation in the cyclophosphamide (CYP)-induced mouse cystitis. Male C57BL/6 [wild-type, (WT)] and/or TLR4
K A Reynolds et al.
Journal of bacteriology, 174(12), 3850-3854 (1992-06-01)
A novel NADPH-dependent enoyl reductase, catalyzing the conversion of 1-cyclohexenylcarbonyl coenzyme A (1-cyclohexenylcarbonyl-CoA) to cyclohexylcarbonyl-CoA, was purified to homogeneity from Streptomyces collinus. This enzyme, a dimer with subunits of identical M(r) (36,000), exhibits a Km of 1.5 +/- 0.3 microM
Xin Xie et al.
Carbohydrate polymers, 225, 115223-115223 (2019-09-16)
A polysaccharide isolated from Strongylocentrotus nudus eggs (SEP) reportedly displays immune activity in vivo. Here, its effect and underlying mechanism in the treatment of pancreatic cancer were investigated. SEP obviously inhibited pancreatic cancer growth by activating NK cells in vitro/vivo
M S Elshahed et al.
Applied and environmental microbiology, 67(4), 1728-1738 (2001-04-03)
The metabolism of benzoate, cyclohex-1-ene carboxylate, and cyclohexane carboxylate by "Syntrophus aciditrophicus" in cocultures with hydrogen-using microorganisms was studied. Cyclohexane carboxylate, cyclohex-1-ene carboxylate, pimelate, and glutarate (or their coenzyme A [CoA] derivatives) transiently accumulated during growth with benzoate. Identification was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service