All Photos(1)
About This Item
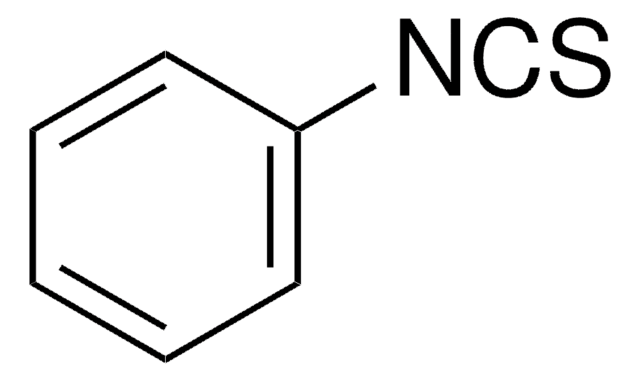
Linear Formula:
O2NC6H4NCS
CAS Number:
Molecular Weight:
180.18
Beilstein:
640027
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
bp
137-138 °C/11 mmHg (lit.)
mp
110-112 °C (lit.)
functional group
isothiocyanate
nitro
SMILES string
[O-][N+](=O)c1ccc(cc1)N=C=S
InChI
1S/C7H4N2O2S/c10-9(11)7-3-1-6(2-4-7)8-5-12/h1-4H
InChI key
NXHSSIGRWJENBH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The solid-state reactivity of hydrazine hydrochloride with 4-nitrophenyl isothiocyanate was studied.
Application
4-Nitrophenyl isothiocyanate has been used in the synthesis of:
- 5,6-dimethyl-2-[(4-nitrophenyl)amino]thieno[2,3-d]pyrimidin-4(3H)-one
- 4,5-dimethyl-2-substituted carbamothioylaminothiophene-3-carboxamides
- acyclic triazene or zwitterionic dihydropyrimidinium imidothiolate derivatives
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Solid?state reactivity of the hydrazine?hydroquinone complex.
Kaupp G and Schmeyers J.
Journal of Physical Organic Chemistry, 13(7), 388-394 (2000)
Investigation into the reaction of 2-amino-4, 5-dimethylthiophene-3-carboxamide with iso (and isothio) cyanates under microwave irradiation.
Davoodnia A, et al.
Heteroatom Chem., 20(6), 346-349 (2009)
Nargess Yousefi Limaee et al.
Journal of fluorescence, 30(2), 375-387 (2020-02-23)
Fluorescent molecularly imprinted polymer (FMIP) optosensor was utilized for the selective identification of 2,4-dichlorophenoxacetic acid (2,4-D) due to worldwide pollution caused by using herbicides in agricultural industry. In this regards, two derivatives of polymerizable 1,8-naphthalimide namely, 1,8-naphthalimide containing thiourea (NI)
Exploring the nucleophilicity of N, N'-diamidocarbenes: Heteroallenes and related compounds as coupling reagents.
Lee YG, et al.
Journal of Physical Organic Chemistry, 25(11), 1027-1027 (2012)
Junta Sano et al.
Polymers, 12(5) (2020-05-24)
Novel interpenetrating polymer networks (IPNs) were synthesized from N-isopropylacrylamide (NIPAM) and polysiloxanes containing a urea or thiourea side group, in addition to the silanol residue, through two reactions, such as the radical gelation of NIPAM and the condensation of silanols
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service