All Photos(1)
About This Item
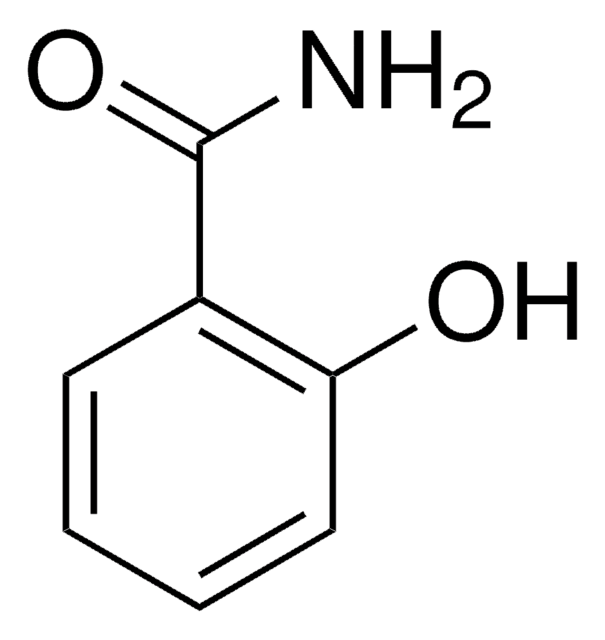
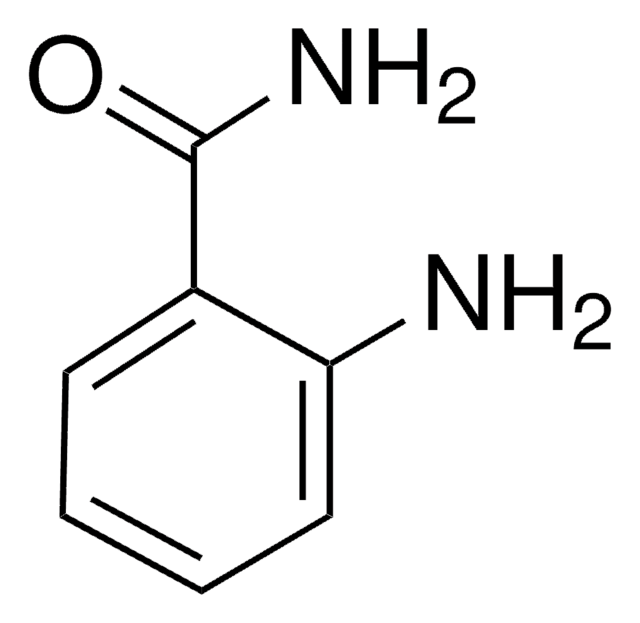
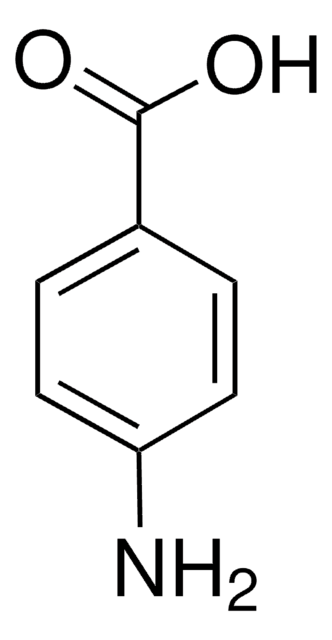
Linear Formula:
HOC6H4CONH2
CAS Number:
Molecular Weight:
137.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
161-162 °C (lit.)
functional group
amide
SMILES string
NC(=O)c1ccc(O)cc1
InChI
1S/C7H7NO2/c8-7(10)5-1-3-6(9)4-2-5/h1-4,9H,(H2,8,10)
InChI key
QXSAKPUBHTZHKW-UHFFFAOYSA-N
Related Categories
General description
The standard molar enthalpy of formation of 4-hydroxybenzamide was studied by micro- or macrocombustion calorimetry.
Application
4-Hydroxybenzamide was used in the synthesis of balanol, a potent protein kinase C (PKC) inhibitor.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R S Randad et al.
Bioorganic & medicinal chemistry, 4(9), 1471-1480 (1996-09-01)
A combination of structure-activity studies, kinetic analysis, X-ray crystallographic analysis, and modeling were employed in the design of a novel series of HIV-1 protease (HIV PR) inhibitors. The crystal structure of a complex of HIV PR with SRSS-2,5-bis[N-(tert-butyloxycarbonyl)amino]-3,4-dihydroxy-1, 6-diphenylhexane (1)
G D Hartman et al.
Bioorganic & medicinal chemistry letters, 9(6), 863-868 (1999-04-17)
A new series of potent, linearly-minimized, orally active, selective GPIIb/IIIa inhibitors is identified. Thus 15 (L-750,034) achieves interaction via a constrained, non-turned conformation that maintains the proper distance between its charged termini and full sulfonamide exosite interaction. The diminutive stature
C Emoto et al.
Xenobiotica; the fate of foreign compounds in biological systems, 37(12), 1408-1420 (2007-10-19)
CJ-036878, N-(3-phenethoxybenzyl)-4-hydroxybenzamide, was developed as an antagonist of the N-methyl-D-aspartate receptor NR2B subunit. Two dimeric metabolites, CJ-047710 and CJ-047713, were identified from the incubation mixture with CJ-036878 in human liver microsomes (HLM). The identification of the enzymes involved in the
Carlos E S Bernardes et al.
The journal of physical chemistry. A, 112(40), 10029-10039 (2008-09-13)
The energetics of the phenolic O-H bond in a series of 2- and 4-HOC 6H 4C(O)Y (Y = H, CH3, CH 2CH=CH2, C[triple bond]CH, CH2F, NH2, NHCH 3, NO2, OH, OCH3, OCN, CN, F, Cl, SH, and SCH3) compounds and
G D Smith et al.
Protein science : a publication of the Protein Society, 5(8), 1502-1511 (1996-08-01)
The structure of a symmetric T3R3f insulin hexamer, complexed with 4-hydroxybenzamide, has been determined using X-ray crystallographic techniques. Data were measured from six crystals grown in microgravity to a resolution of 1.4 A and the structure has been refined including
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service