245100
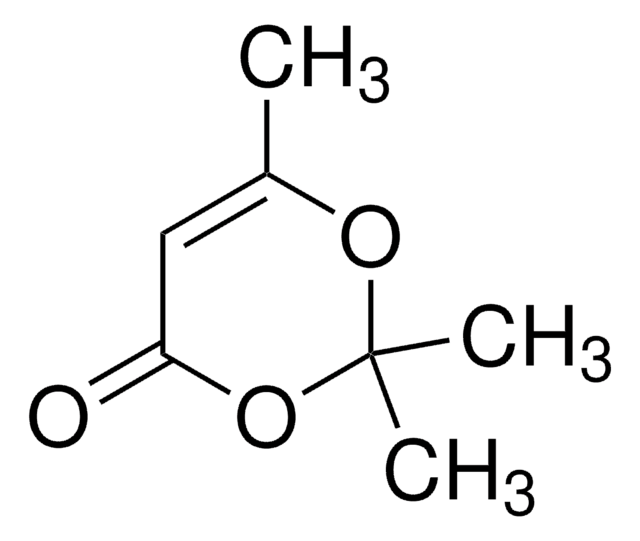
2,2,6-Trimethyl-4H-1,3-dioxin-4-one
95%
Synonym(s):
Diketene acetone adduct
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H10O3
CAS Number:
Molecular Weight:
142.15
Beilstein:
2408
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.460 (lit.)
bp
~275 °C (lit.)
65-67 °C/2 mmHg (lit.)
mp
12-13 °C (lit.)
solubility
water: insoluble
density
1.07 g/mL at 25 °C (lit.)
functional group
ester
ether
ketal
SMILES string
CC1=CC(=O)OC(C)(C)O1
InChI
1S/C7H10O3/c1-5-4-6(8)10-7(2,3)9-5/h4H,1-3H3
InChI key
XFRBXZCBOYNMJP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2,2,6-Trimethyl-4H-1,3-dioxin-4-one is used as a building block in organic synthesis and serves as a direct precursor to β-dicarbonyl compounds
Application
2,2,6-Trimethyl-4H-1,3-dioxin-4-one was used in the synthesis of acetylketene by flash pyrolysis.
Other Notes
remainder acetone
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
57.2 °F - closed cup
Flash Point(C)
14 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
David M. Birney et al.
The Journal of organic chemistry, 62(21), 7114-7120 (2001-10-24)
Acetylketene (1) was generated by flash pyrolysis of 2,2,6-trimethyl-4H-1,3-dioxin-4-one (6). The selectivities of 1 toward a number of representative functional groups were measured for the first time in a series of competitive trapping reactions. The trend in reactivities toward 1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service