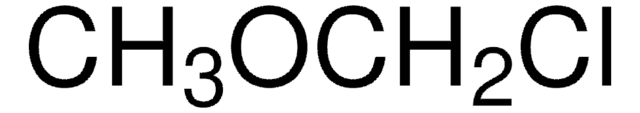242349
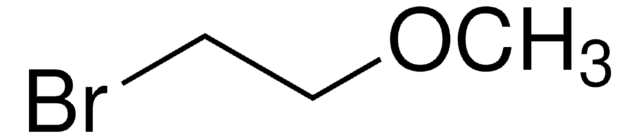
2-Chloroethyl methyl ether
98%
Synonym(s):
2-Methoxyethyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClCH2CH2OCH3
CAS Number:
Molecular Weight:
94.54
Beilstein:
1731028
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.408 (lit.)
bp
89-90 °C (lit.)
density
1.035 g/mL at 25 °C (lit.)
functional group
chloro
ether
SMILES string
COCCCl
InChI
1S/C3H7ClO/c1-5-3-2-4/h2-3H2,1H3
InChI key
XTIGGAHUZJWQMD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Chloroethyl methyl ether (2-Methoxyethyl chloride) was used in the synthesis of acyclic nucleosides of thieno[2,3-d]pyrimidine derivatives.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
46.4 °F - closed cup
Flash Point(C)
8 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nasser A Hassan et al.
Nucleosides, nucleotides & nucleic acids, 26(4), 379-390 (2007-05-05)
The reaction of compounds 1, 2, 3, 4, or 13 with 2-chloroethyl methyl ether or 2,3,4,6-tetra-O-acetyl-alpha-D-glucopyranosyl bromide, afforded some acyclic and cyclic nucleosides of thieno[2,3-d]pyrimidine derivatives. Furthermore, cyclic C-nucleosides 24 and 25 were prepared from the reaction of 20, 21
P T K Lee et al.
Dalton transactions (Cambridge, England : 2003), 46(27), 8818-8826 (2017-04-01)
B(C
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service