All Photos(1)
About This Item
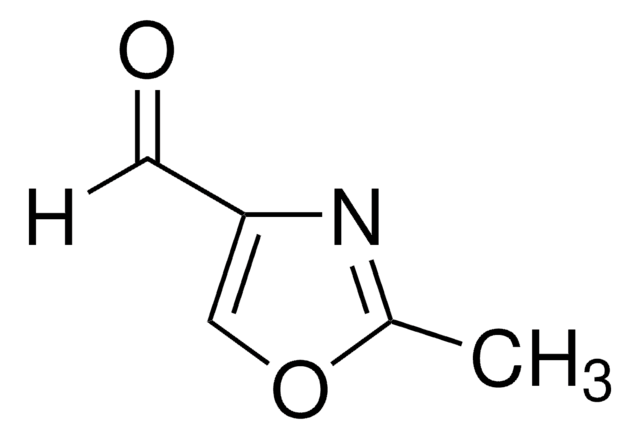
Empirical Formula (Hill Notation):
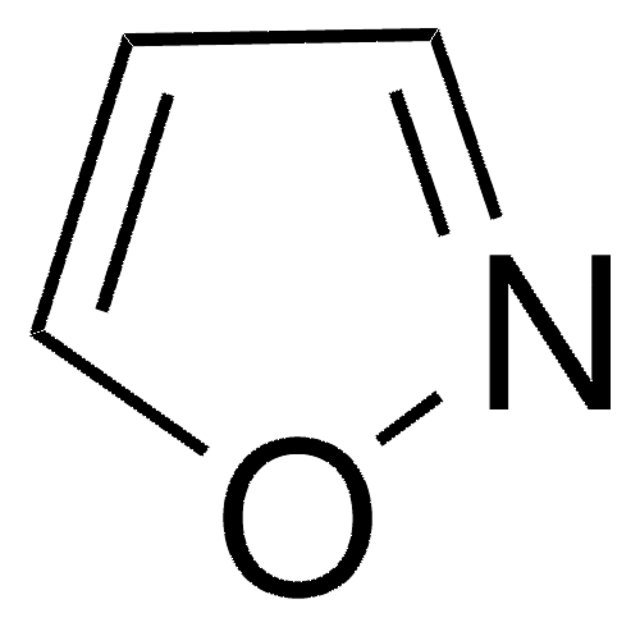
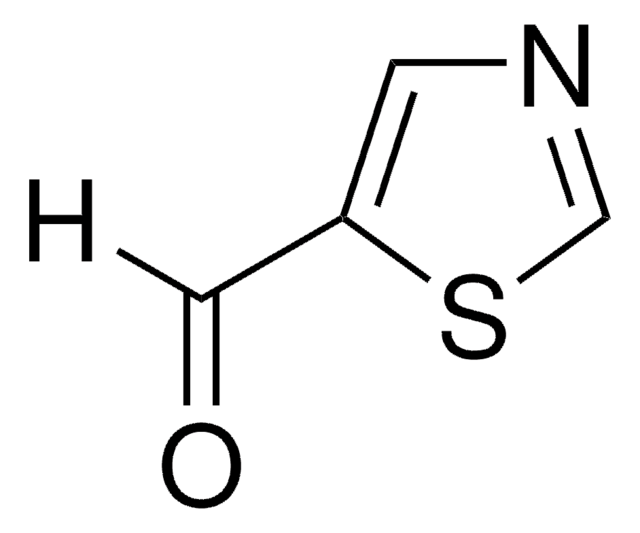
C3H3NO
CAS Number:
Molecular Weight:
69.06
Beilstein:
103851
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.425 (lit.)
bp
69-70 °C (lit.)
mp
−87-−84 °C (lit.)
density
1.05 g/mL at 25 °C (lit.)
SMILES string
c1cocn1
InChI
1S/C3H3NO/c1-2-5-3-4-1/h1-3H
InChI key
ZCQWOFVYLHDMMC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Oxazole is the parent molecule for a large class of heterocyclic aromatic compounds. It is a weak base that can be used as an electron-deficient diene in the Diels-Alder cycloaddition reaction. It undergoes nitration, sulfonation, halogenation, Friedel-Crafts alkylation, and acylation.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
66.2 °F - closed cup
Flash Point(C)
19 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Maryna V Kachaeva et al.
Computational biology and chemistry, 74, 294-303 (2018-04-27)
Based on modern literature data about biological activity of E7010 derivatives, a series of new sulfonamides as potential anticancer drugs were rationally designed by QSAR modeling methods Сlassification learning QSAR models to predict the tubulin polymerization inhibition activity of novel
Haseen Ahmad et al.
European journal of medicinal chemistry, 208, 112759-112759 (2020-09-05)
Oxazole derivatives are important medicinal compounds which are inhibitors of various enzymes such as NPP1, NPP2, NPP3, tyrosine kinase, dipeptidyl-peptidase IV, cyclooxygenase-2, and protein tyrosine phosphatase. In this study, an extensive range of new biologically active biphenyl oxazole derivatives was
Dawid Siodłak et al.
The journal of physical chemistry. B, 118(9), 2340-2350 (2014-02-18)
Oxazole ring occurs in numerous natural peptides, but conformational properties of the amino acid residue containing the oxazole ring in place of the C-terminal amide bond are poorly recognized. A series of model compounds constituted by the oxazole-amino acids occurring
Ilya Lyagin et al.
Molecules (Basel, Switzerland), 24(13) (2019-06-30)
Mycotoxins are highly dangerous natural compounds produced by various fungi. Enzymatic transformation seems to be the most promising method for detoxification of mycotoxins. This review summarizes current information on enzymes of different classes to convert various mycotoxins. An in-depth analysis
Kristjan Bloudoff et al.
Proceedings of the National Academy of Sciences of the United States of America, 114(1), 95-100 (2016-12-21)
Nonribosomal peptide synthetases (NRPSs) are a family of multidomain, multimodule enzymes that synthesize structurally and functionally diverse peptides, many of which are of great therapeutic or commercial value. The central chemical step of peptide synthesis is amide bond formation, which
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service