22110
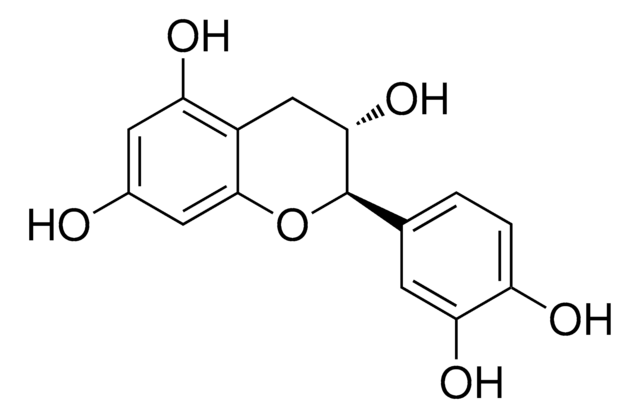
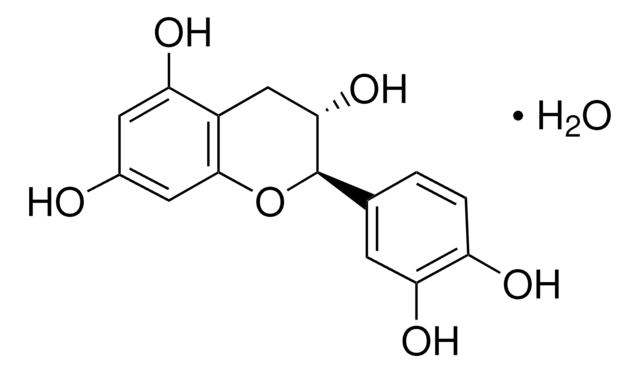
(+)-Catechin hydrate
≥96.0% (sum of enantiomers, HPLC)
Synonym(s):
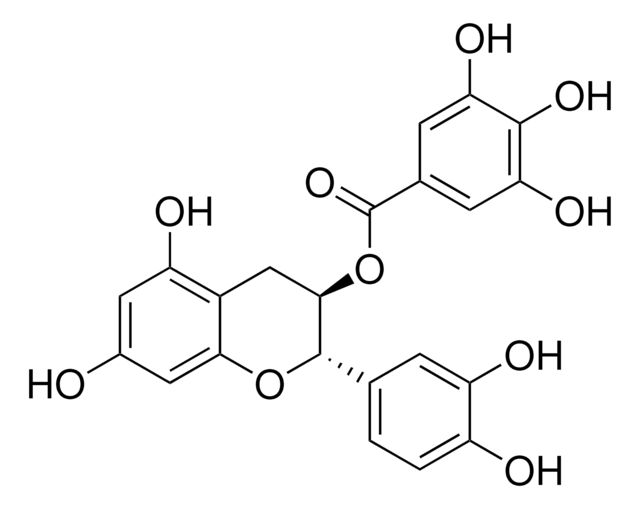
(+)-Cyanidol-3, (2R,3S)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol
About This Item
Recommended Products
Assay
≥96.0% (sum of enantiomers, HPLC)
form
powder
optical activity
[α]/D +26±2°, c = 1 in H2O
impurities
≤8% water
mp
175-177 °C (anhydrous) (lit.)
storage temp.
2-8°C
SMILES string
[H]O[H].O[C@H]1Cc2c(O)cc(O)cc2O[C@@H]1c3ccc(O)c(O)c3
InChI
1S/C15H14O6.H2O/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7;/h1-5,13,15-20H,6H2;1H2/t13-,15+;/m0./s1
InChI key
OFUMQWOJBVNKLR-NQQJLSKUSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- As an inhibitor of steel corrosion in hydrochloric acid solution.
- As a model compound in the study of antimicrobial activities of flavonoids on Escherichia coli.
- As a starting material for the synthesis of catechin glucosides of biological importance.
Caution
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Coumaric acid; Quercitrin; Myricetin; Quercetin
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Protocols
Coumaric acid; Quercitrin; Myricetin; Quercetin
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service