219355
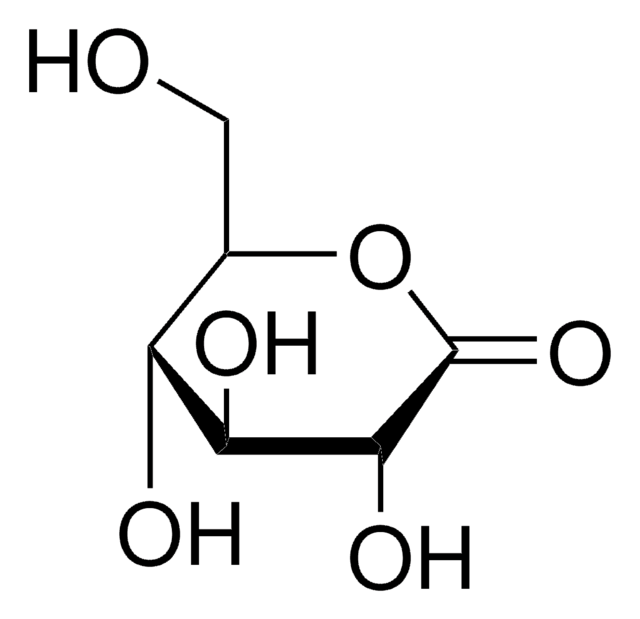
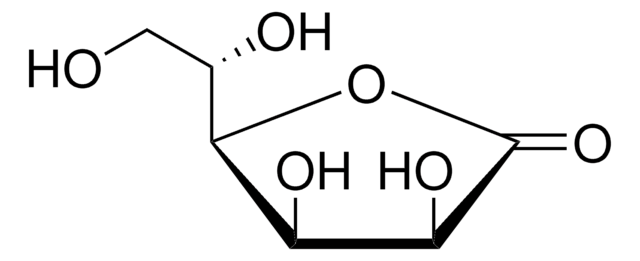
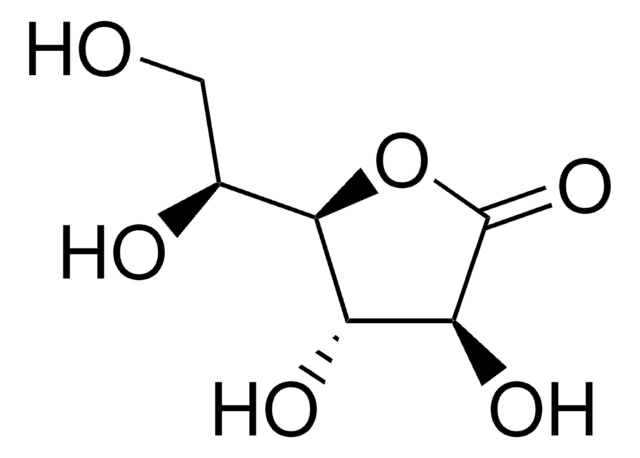
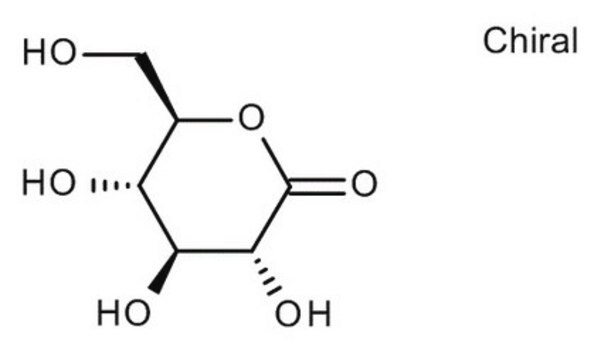
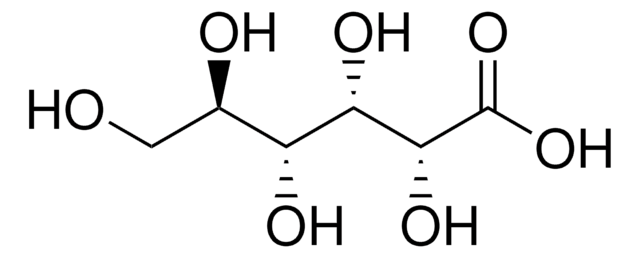
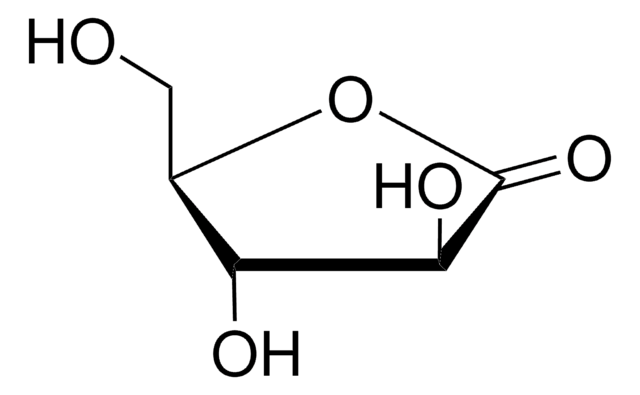
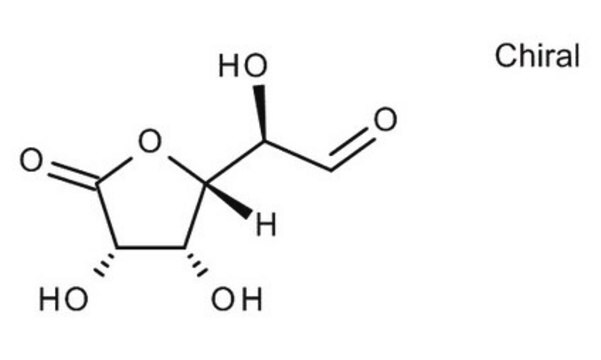
D-Gulonic acid γ-lactone
97%
Synonym(s):
D-(−)-Gulono-1,4-lactone, D-Gulono γ-lactone, D-Gulono-1,4-lactone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H10O6
CAS Number:
Molecular Weight:
178.14
Beilstein:
83012
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
optical activity
[α]20/D −55°, c = 4 in H2O
mp
182-188 °C (lit.)
storage temp.
2-8°C
SMILES string
OC[C@@H](O)[C@@H]1OC(=O)[C@H](O)[C@@H]1O
InChI
1S/C6H10O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2-5,7-10H,1H2/t2-,3+,4-,5+/m1/s1
InChI key
SXZYCXMUPBBULW-LECHCGJUSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ayodele O Olabisi et al.
The Journal of organic chemistry, 70(17), 6782-6789 (2005-08-13)
A convenient method to obtain unknown chiral C2- and C3-functionalized aldono-1,4-lactone derivatives starting from l-ascorbic acid, which would be valuable in the synthesis of derivatives of various pharmacologically active agents for structure-activity studies, is described. The practicality of this approach
Marjan Jeselnik et al.
Organic letters, 5(15), 2651-2653 (2003-07-19)
[reaction: see text] A new synthesis of L-noviose (11), a sugar moiety of novobiocin, is presented. D-Gulonolactone was initially converted in a few steps to the key ester derivative 7 [1-O-benzyl methyl 2,3-O-(1-methylethylidene)-alpha-L-lyxofuranosiduronate]. An appropriate selection of protecting groups enabled
G W Fleet et al.
Carbohydrate research, 205, 269-282 (1990-09-19)
The synthesis of the enantiomers of 6-epicastanospermine and of 1,6-diepicastanospermine from the enantiomeric gulonolactones is reported and the structure of the former is established as (1S,6R,7R,8R,8aR)-1,6,7,8-tetrahydroxyoctahydroindolizine. The inhibitory activities of the diastereomers against the amyloglucosidase-catalysed hydrolysis of p-nitrophenyl alpha-D-glucopyranoside were
F Puskás et al.
FEBS letters, 430(3), 293-296 (1998-08-04)
The orientation of gulonolactone oxidase activity was investigated in rat liver microsomes. Ascorbate formation upon gulonolactone addition resulted in higher intravesicular than extravesicular ascorbate concentrations in native microsomal vesicles. The intraluminal ascorbate accumulation could be prevented or the accumulated ascorbate
A Krasnov et al.
Genetic analysis : biomolecular engineering, 15(3-5), 115-119 (1999-12-22)
The reviewed studies addressed the possibility of using gene transfer for correction of L-ascorbic acid biosynthesis and carbohydrate utilization in rainbow trout. Analyses of enzymatic activities in the L-AAB pathway indicated that reasons for the lack of L-AA production can
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service