20021
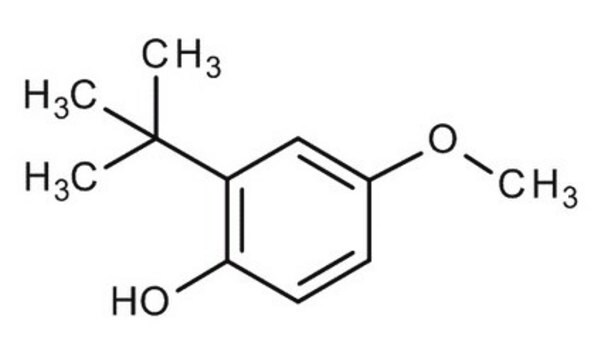
3-tert-Butyl-4-hydroxyanisole
≥98% (sum of isomers, GC), ≤10% 2-BHA basis (GC)
Synonym(s):
2-tert-Butyl-4-methoxyphenol, 3-BHA, BHA
About This Item
Recommended Products
Quality Level
Assay
≥98% (sum of isomers, GC)
form
solid
composition
2-BHA, ≤10% GC
3-BHA, ≥90% GC
impurities
≤1% 4-hydroxyanisole
ign. residue
≤0.05%
mp
48-63 °C
solubility
ethanol: soluble 1 g/10 mL, clear, colorless to faint yellow or tan
SMILES string
COc1ccc(O)c(c1)C(C)(C)C
InChI
1S/C11H16O2/c1-11(2,3)9-7-8(13-4)5-6-10(9)12/h5-7,12H,1-4H3
InChI key
MRBKEAMVRSLQPH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Regulation of Smad signaling in mesenchymal stem cells: 3-tert-Butyl-4-hydroxyanisole disrupts the differentiation of C3H10T1/2 mesenchymal stem cells into brown adipocytes by modulating Smad signaling pathways, with potential implications for obesity and metabolic syndrome research (Wang et al., 2023).
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service