199877
Lithium aluminum hydride
powder, reagent grade, 95%
Synonym(s):
LAH, Lithium alanate, Lithium tetrahydroaluminate
About This Item
Recommended Products
grade
reagent grade
Quality Level
Assay
95%
form
powder
reaction suitability
reagent type: reductant
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
125 °C (dec.) (lit.)
greener alternative category
SMILES string
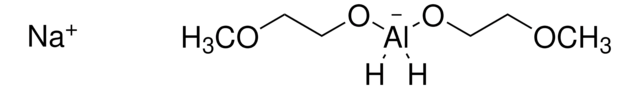
[Li].[AlH3]
InChI
1S/Al.Li.4H/q-1;+1;;;;
InChI key
OCZDCIYGECBNKL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
LiAlH4 is a promising substance for hydrogen storage applications. Its properties include high gravimetric and volumetric hydrogen densities . It can also be used as a reducing agent in the preparation of reduced graphene oxide (rGO).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1A - Water-react 1
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Our research impacts on the hydrogen energy economy through the development of “smart” nanofilms for the protection of metal hydrides against air and moisture, while permitting release of hydrogen gas through these semi permeable nanofilms.
Mechanochemical Effect of Severe Plastic Deformations: Metal Alloys, Hydrides and Molecular Solids
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service