184608
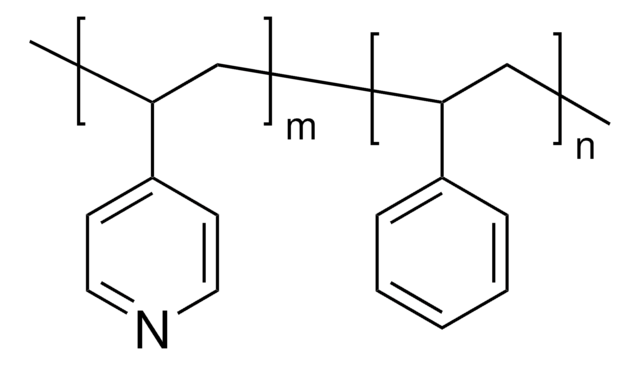
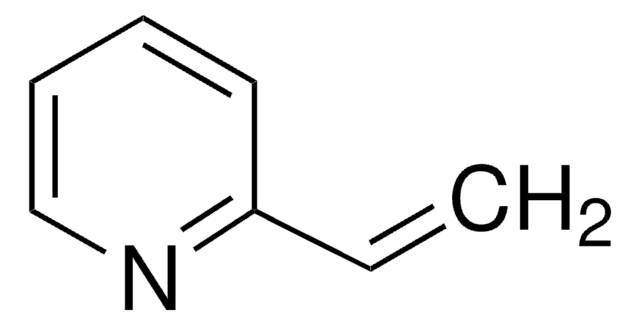
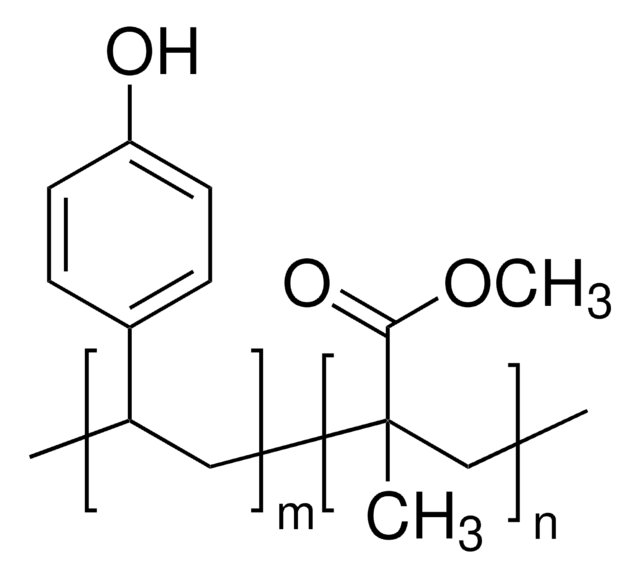
Poly(2-vinylpyridine-co-styrene)
average Mn ~130,000, average Mw ~220,000 by LS, granular
Synonym(s):
2-Vinylpyridine-styrene copolymer, Styrene-2-vinylpyridine copolymer, Vinylbenzene-2-vinylpyridine copolymer
About This Item
Recommended Products
form
granular
mol wt
average Mn ~130,000
average Mw ~220,000 by LS
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
greener alternative category
, Enabling
General description
Application
Packaging
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Advances in the electrochemical conversion of water to and from hydrogen and oxygen have principally been achieved through the development of new materials and by understanding the mechanisms of the degradation of proton exchange membrane fuel cells (PEMFC) during operation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service