All Photos(1)
About This Item
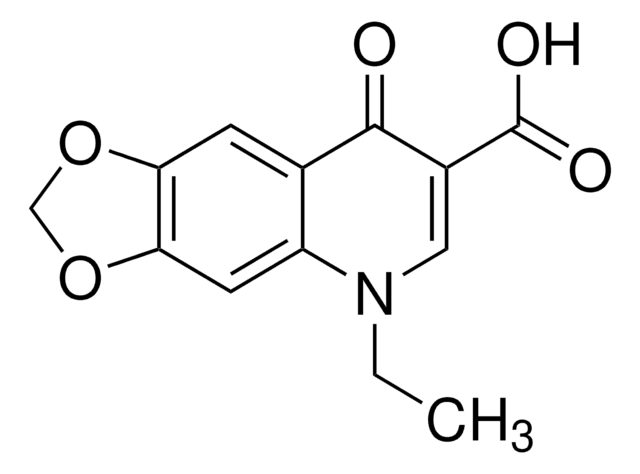
Empirical Formula (Hill Notation):
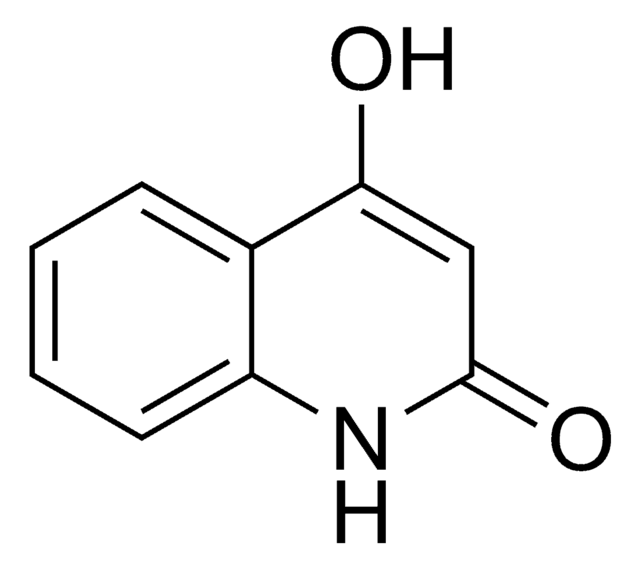
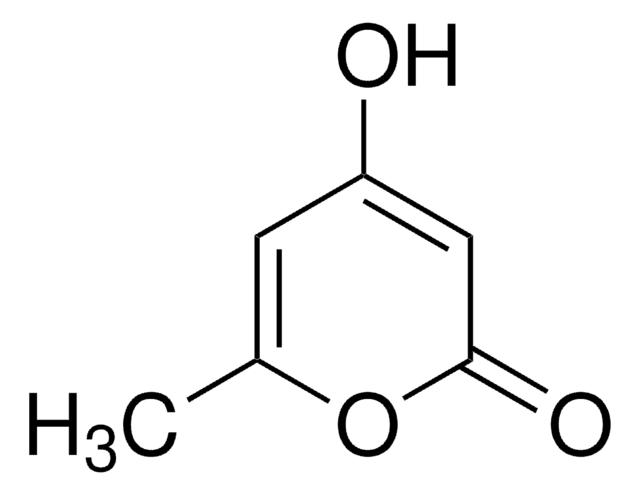
C10H9NO2
CAS Number:
Molecular Weight:
175.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
269-271 °C (lit.)
SMILES string
CN1C(=O)C=C(O)c2ccccc12
InChI
1S/C10H9NO2/c1-11-8-5-3-2-4-7(8)9(12)6-10(11)13/h2-6,12H,1H3
InChI key
RTNPPPQVXREFKX-UHFFFAOYSA-N
Application
4-Hydroxy-1-methyl-2(1H)-quinolone was used as nucleophile in electrochemical oxidation of catechols and the reaction was studied by cyclic voltammetry and controlled-potential coulometry. It was used in ceric ammonium nitrate-mediated oxidative cycloaddition of 1,3-dicarbonyls to conjugated compounds to yield substituted dihydrofurans, dihydrofurocoumarins, dihydrofuroquinolinones and dihydrofurophenalenones. It was used in the synthesis of :
- 3-(4-methoxybenzyl)-4-hydroxy-1-methylquinolinone
- 3-(benzo[d][1,3]dioxol-5-ylmethyl)-4-hydroxy-1-methylquinolin-2(1H)-one
- 3-(4-chlorobenzyl)-4-hydroxy-1-methylquinolin-2(1H)-one
- 3,3′-bis(4-chlorobenzylidene)-1,10-methylquinolin-2,20-(1H)-one
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mechanistic study of electrochemical oxidation of catechols in the presence of 4-hydroxy-1-methyl-2 (1H)-quinolone: Application to the electrochemical synthesis.
Fakhari AR, et al.
Electrochimica Acta, 50(27), 5322-5328 (2005)
Ceric ammonium nitrate (CAN)-mediated oxidative cycloaddition of 1, 3-dicarbonyls to conjugated compounds. Efficient synthesis of dihydrofurans, dihydrofurocoumarins, dihydrofuroquinolinones, dihydrofurophenalenones, and furonaphthoquinone natural products.
Lee YR, et al.
Tetrahedron, 56(45), 8845-8853 (2000)
Iridium catalysed alkylation of 4-hydroxy coumarin, 4-hydroxy-2-quinolones and quinolin-4 (1H)-one with alcohols under solvent free thermal conditions.
Grigg R, et al.
Tetrahedron, 65(36), 7468-7473 (2009)
Michael B Hicks et al.
The Analyst, 142(3), 525-536 (2017-01-18)
The use of a coulometric array detector in tandem with HPLC-UV was evaluated for the absolute quantitation of pharmaceutical compounds without standards, an important capability gap in contemporary pharmaceutical research and development. The high-efficiency LC flow-through electrochemical detector system allows
Mohankumar Saraladevi Resmi et al.
FEBS letters, 589(3), 332-341 (2015-01-06)
Type III polyketide synthases (PKSs) catalyze the biosynthesis of various medicinally important secondary metabolites in plants, but their role in growth and stress response is unclear. Here, we overexpressed quinolone synthase (QNS) from bael in tobacco. QNS-overexpressing plants showed an
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service