132004
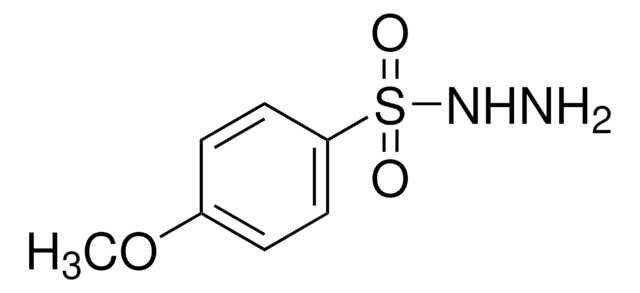
p-Toluenesulfonyl hydrazide
97%
Synonym(s):
p-Toluenesulfonhydrazide, p-Toluenesulfonic acid hydrazide, p-Toluenesulfonyl hydrazide, Tosylhydrazide
About This Item
Recommended Products
Assay
97%
form
powder
mp
103-108 °C (lit.)
functional group
hydrazine
SMILES string
Cc1ccc(cc1)S(=O)(=O)NN
InChI
1S/C7H10N2O2S/c1-6-2-4-7(5-3-6)12(10,11)9-8/h2-5,9H,8H2,1H3
InChI key
ICGLPKIVTVWCFT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 2 - Self-react. D
Supplementary Hazards
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 14501-1G | 4061838736062 |
| 14502-1G | 4061826652312 |
| 14504-1G-F | 4061826652329 |
| 14504-250MG-F | 4061838736086 |
| 14508-1G | 4061826652336 |
| 14509S-1KG-R | 4061824069440 |
| 909149-1G | |
| 752452-5G | 4061832907291 |
| 752460-1G | 4061832907307 |
| 753084-1G | 4061837889585 |
| 14501-250MG | 4061833278727 |
| 14502-250MG | 4061833278734 |
| 14508-250MG | |
| 14509-1G-F | 4061826652343 |
| 14509-250MG-F | |
| 753084-5G | 4061832907543 |
| 752444-1G | 4061832907277 |
| 752452-1G | 4061832907284 |
| 752444-5G | 4061833600603 |
| 752460-5G | 4061833600610 |
| P9906-1G | 4061838355577 |
| P9906-5G | 4061833612903 |
| 132004-100G | 4061838728722 |
| 132004-500G | 4061833544433 |
| 132004-25G | 4061838728739 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1H-1,2,3-Triazolo[4,5-b]pyridine 98%](/deepweb/assets/sigmaaldrich/product/structures/344/744/1e7fa2cf-1258-48e0-909f-92509981f43d/640/1e7fa2cf-1258-48e0-909f-92509981f43d.png)