120227
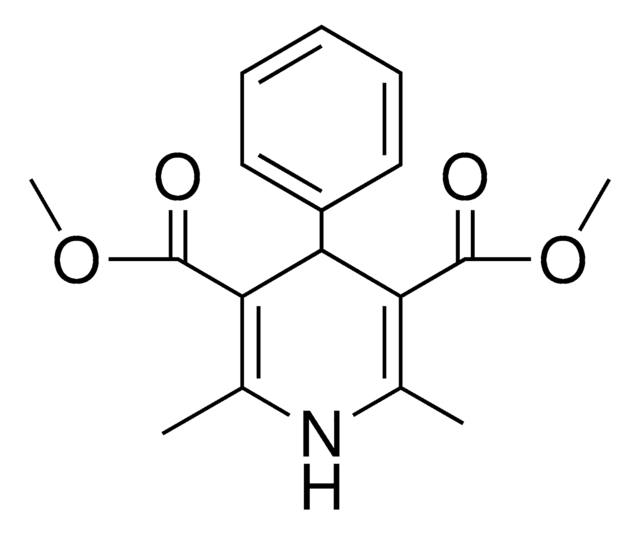
Diethyl 1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate
95%
Synonym(s):
Hantzsch ester
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C13H19NO4
CAS Number:
Molecular Weight:
253.29
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
mp
178-183 °C (lit.)
solubility
organic solvents: soluble
functional group
ester
SMILES string
CCOC(=O)C1=C(C)NC(C)=C(C1)C(=O)OCC
InChI
1S/C13H19NO4/c1-5-17-12(15)10-7-11(13(16)18-6-2)9(4)14-8(10)3/h14H,5-7H2,1-4H3
InChI key
LJXTYJXBORAIHX-UHFFFAOYSA-N
Related Categories
General description
Diethyl 1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate is often used as a building block in organic synthesis for the preparation of various biologically active compounds.
Application
Diethyl 1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate (DTP) was used to study the mechanism of electrochemical oxidation of DTP in ethanol/water solutions on a glassy carbon electrode.
Used as a hydrogen source in organocatalytic reductive amination and conjugate reduction.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
[Geroprotective activity of 2,6-dimethyl-3,5-diethoxycarbonyl-l,4-dihydropyridine].
N M Emanuél' et al.
Doklady Akademii nauk SSSR, 284(5), 1271-1274 (1985-01-01)
M V Bilenko et al.
Biulleten' eksperimental'noi biologii i meditsiny, 96(9), 8-11 (1983-09-01)
Prophylactic injection of the natural antioxidant alpha-tocopherol and synthetic antioxidants ionol, diludin and 6-mercurascan prevented the development of lesions during acute renal ischemia and subsequent reperfusion. Ionol proved more effective on intraperitoneal injection than on intragastric route of administration. It
M Mamadiev et al.
Voprosy meditsinskoi khimii, 29(2), 83-89 (1983-03-01)
Appearance of cadaverine deaminating activity in mitochondrial fractions of liver and kidney of rabbits with experimental alimentary hypercholesterolaemia was prevented by an antioxant diludin (2,6-dimethyl-3,5-diethoxycarbonyl-1,4-dihydropyridine) which also decreased the abnormally elevated AMP-deaminating activity and elevated the decreased monoamine oxidase activity
Jing Zhang et al.
iScience, 23(1), 100755-100755 (2019-12-31)
The alkoxyl radical is an essential reactive intermediate in mechanistic studies and organic synthesis with hydrogen atom transfer (HAT) reactivity. However, compared with intramolecular 1,5-HAT or intermolecular HAT of alkoxyl radicals, the intramolecular 1,2-HAT reactivity has been limited to theoretical
N A Basova et al.
Rossiiskii fiziologicheskii zhurnal imeni I.M. Sechenova, 88(5), 650-657 (2002-07-26)
The main concern of this work was to examine the relation between altered antioxidant status on the one hand and increase in L-tryptophan absorption in the small intestine in order to bring further information regarding to possible role of vitamin
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service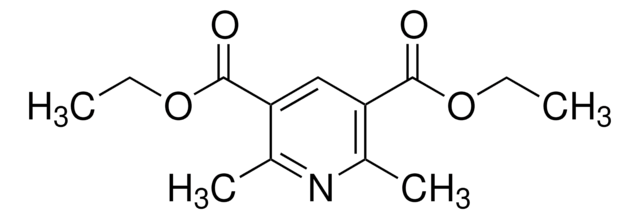



![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
![Tris[2-phenylpyridinato-C2,N]iridium(III) sublimed grade](/deepweb/assets/sigmaaldrich/product/structures/167/234/658d0b76-d31d-4fd5-8041-e04e207227c9/640/658d0b76-d31d-4fd5-8041-e04e207227c9.png)