Alle Fotos(1)
Wichtige Dokumente
T8019
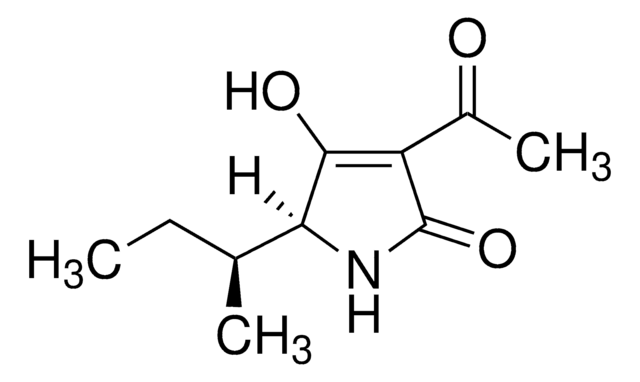
Tentoxin from Alternaria tenuis
Naturally occurring phytotoxic cyclic tetrapeptide
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C22H30N4O4
CAS-Nummer:
Molekulargewicht:
414.50
MDL-Nummer:
UNSPSC-Code:
12352200
PubChem Substanz-ID:
NACRES:
NA.56
Empfohlene Produkte
Biologische Quelle
fungus (Alternaria tenuis)
Qualitätsniveau
Assay
≥95% (HPLC)
Lagertemp.
2-8°C
SMILES String
CC(C)CC1NC(=O)C(C)N(C)C(=O)CNC(=O)\C(=C/c2ccccc2)N(C)C1=O
InChI
1S/C22H30N4O4/c1-14(2)11-17-22(30)26(5)18(12-16-9-7-6-8-10-16)21(29)23-13-19(27)25(4)15(3)20(28)24-17/h6-10,12,14-15,17H,11,13H2,1-5H3,(H,23,29)(H,24,28)/b18-12-/t15-,17-/m0/s1
InChIKey
SIIRBDOFKDACOK-LFXZBHHUSA-N
Allgemeine Beschreibung
Tentoxin is a majorly occurring cyclic tetrapeptide mycotoxin that is excreted by the Alternaria fungal species like Alternaria alternata and Alternaria tenuis.
Biochem./physiol. Wirkung
It induces chlorosis in germinating seedlings of many dicotyledonous plants. Tentoxin has been postulated to inhibit cyclic photophosphorylation by acting as an energy transfer inhibitor at the terminal steps of ATP synthesis and to target the F1 moiety of photosynthetic H+-ATPases. A kinetic analysis of the action of tentoxin on the chloroplast F1 H+-ATPase (CF1) portion of chloroplast ATP synthase has been reported.
Tentoxin is phytotoxic, which induces chlorosis in plant leaves and germinating seedlings. It also halts photophosphorylation by inhibiting chloroplastic ATP synthase. Tentoxin over energizes thylakoids and is converted to isotentoxin by UV irradiation.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Inhibition of photophosphorylation by tentoxin, a cyclic tetrapeptide.
C J Arntzen
Biochimica et biophysica acta, 283(3), 539-542 (1972-12-14)
C Sigalat et al.
FEBS letters, 368(2), 253-256 (1995-07-17)
The effect of tentoxin at high concentrations was investigated in thylakoids and proteoliposomes containing bacteriorhodopsin and CF0CF1. Venturicidin-sensitive ATP hydrolysis, ATP-generated delta pH and ATP synthesis were practically 100% inhibited at 2 microM tentoxin, and restored to various extents beyond
Nicolas Loiseau et al.
Journal of peptide science : an official publication of the European Peptide Society, 8(7), 335-346 (2002-08-01)
Tentoxin is a naturally occurring phytotoxic cyclic tetrapeptide excreted by fungi of the Alternaria alternata family. The four total syntheses of tentoxin published to date give poor total yields, mainly owing to two difficulties, the introduction of the dehydro amino
Georg Groth
Proceedings of the National Academy of Sciences of the United States of America, 99(6), 3464-3468 (2002-03-21)
Tentoxin, a natural cyclic tetrapeptide produced by phytopathogenic fungi from the Alternaria species affects the catalytic function of the chloroplast F(1)-ATPase in certain sensitive species of plants. In this study, we show that the uncompetitive inhibitor tentoxin binds to the
Georg Groth et al.
The Journal of biological chemistry, 277(23), 20117-20119 (2002-04-12)
In contrast to the homologous bacterial and mitochondrial enzymes the chloroplast F(1)-ATPase (CF(1)) is strongly affected by the phytopathogenic inhibitor tentoxin. Based on structural information obtained from crystals of a CF(1)-tentoxin co-complex (Groth, G. (2002) Proc. Natl. Acad. Sci. U.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.