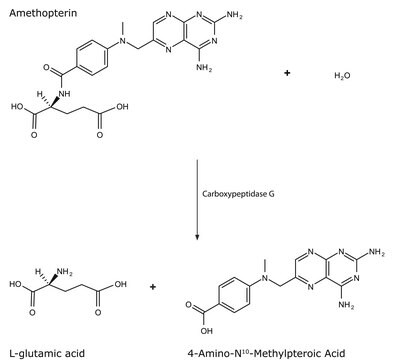
Wichtige Dokumente
SAE0046
Carboxypeptidase A aus Rinderpankreas
(Type II-PMSF treated), ≥20 units/mg protein
Synonym(e):
Carboxypolypeptidase, Peptidyl-L-aminosäure-Hydrolase
About This Item
Empfohlene Produkte
Biologische Quelle
bovine pancreas
Qualitätsniveau
Form
aqueous suspension
Qualität
(Type II-PMSF treated)
Spezifische Aktivität
≥20 units/mg protein
Mol-Gew.
~35 kDa
Aufgereinigt durch
2× crystallization
Verunreinigungen
≤0.05 BTEE units/mg protein chymotrpsin
≤10 BAEE units/mg protein trypsin
Lagertemp.
2-8°C
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
Biochem./physiol. Wirkung
Einheitendefinition
Angaben zur Herstellung
Hinweis zur Analyse
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.