Alle Fotos(1)
Wichtige Dokumente
P8489
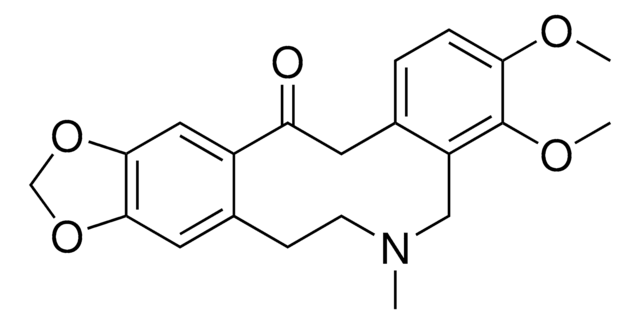
Protopine hydrochloride
≥98%, solid
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C20H19NO5·HCl
CAS-Nummer:
Molekulargewicht:
389.83
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
51111800
PubChem Substanz-ID:
NACRES:
NA.77
Empfohlene Produkte
Qualitätsniveau
Assay
≥98%
Form
solid
Lagertemp.
2-8°C
SMILES String
Cl.CN1CCc2cc3OCOc3cc2C(=O)Cc4ccc5OCOc5c4C1
InChI
1S/C20H19NO5.ClH/c1-21-5-4-13-7-18-19(25-10-24-18)8-14(13)16(22)6-12-2-3-17-20(15(12)9-21)26-11-23-17;/h2-3,7-8H,4-6,9-11H2,1H3;1H
InChIKey
NWNVDSJZGYDVQW-UHFFFAOYSA-N
Allgemeine Beschreibung
Protopine is a celandine alkaloid and a bioactive compound associated with the plant families including fumariaceae, berberidaceae and papaveraceae. It is metabolized by demethylenation in the presence of the cytochrome enzymes cytochrome P450 family 2 subfamily d polypeptide 1 (CYP2D1) and cytochrome P450 family 2 subfamily c polypeptide 11 (CYP2C11).
Anwendung
Protopine hydrochloride may be used in the calibration curve preparation for the quantification of alkaloids from Fumaria capreolata using liquid chromatography coupled to diode array detection and electrospray ionization tandem mass spectrometry (LC-DAD-MS) and tandem mass spectrometry(MS/MS). It may also be used as an alkaloid in cytotoxicity and permeability studies carcinogenic cell lines.
Biochem./physiol. Wirkung
Protopine hydrochloride is a Ca2+ channel blocker and antiplatelet agent.
Protopine possesses anti-parasitic, antimicrobial and anti-inflammatory property. It mediates mitotic arrest by favoring tubulin polymerization. Protopine elicits anti-invasive effects in breast cancer tumor progression.. It also provides protection against oxidative stress-induced cell death.
Vorsicht
vor Licht schützen
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Deok Sung Bae et al.
BMB reports, 45(2), 108-113 (2012-03-01)
Protopine is an isoquinoline alkaloid contained in plants in northeast Asia. In this study, we investigated whether protopine derived from Hypecoum erectum L could suppress lipopolysaccharide (LPS)-induced inflammatory responses in murine macrophages (Raw 264.7 cells). Protopine was found to reduce
Xiao Wang et al.
Journal of chromatography. A, 1115(1-2), 267-270 (2006-04-20)
pH-zone-refining counter-current chromatography was successfully applied to the separation of alkaloids from a crude extract of Corydalis decumbens (Thunb.) Pers. using a multilayer coil planet centrifuge (CPC). The experiment was performed with a two-phase solvent system composed of methyl tert-butyl
David K Liscombe et al.
The Journal of biological chemistry, 282(20), 14741-14751 (2007-03-29)
S-Adenosyl-l-methionine:tetrahydroprotoberberine cis-N-methyltransferase (EC 2.1.1.122) catalyzes the conversion of (S)-stylopine to the quaternary ammonium alkaloid, (S)-cis-N-methylstylopine, as a key step in the biosynthesis of protopine and benzophenanthridine alkaloids in plants. A full-length cDNA encoding a protein exhibiting 45 and 48% amino
Xianghua Xiao et al.
European journal of pharmacology, 591(1-3), 21-27 (2008-07-08)
Calcium and lipid peroxidation play important roles in oxidative stress-induced cellular injury and apoptosis, which ultimately cause cell death. In this study we examined whether protopine had a neuroprotection against H(2)O(2)-induced injury in PC12 cells. Pretreatment of PC12 cells with
Anshu Rathi et al.
Phytomedicine : international journal of phytotherapy and phytopharmacology, 15(6-7), 470-477 (2008-01-01)
The present investigation demonstrates the hepatoprotective potential of 50% ethanolic water extract of whole plant of Fumaria indica and its three fractions viz., hexane, chloroform and butanol against d-galactosamine induced hepatotoxicity in rats. The hepatoprotection was assessed in terms reduction
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.