Alle Fotos(1)
Wichtige Dokumente
M9445
Matrix Metalloproteinase-2 aus mouse
recombinant, expressed in NSO cells, >95% (SDS-PAGE), buffered aqueous glycerol solution
Synonym(e):
72 kD Gelatinase, 72 kD Type IV Collagenase, Gelatinase A, MMP-2
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empfohlene Produkte
Rekombinant
expressed in NSO cells
Qualitätsniveau
Assay
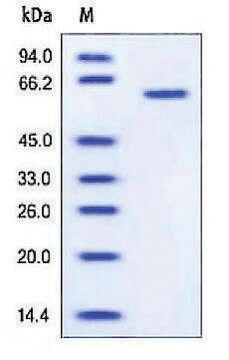
>95% (SDS-PAGE)
Form
buffered aqueous glycerol solution
Mol-Gew.
apparent mol wt ~72 kDa
Versandbedingung
wet ice
Lagertemp.
−20°C
Angaben zum Gen
mouse ... Mmp2(17390)
rat ... Mmp2(81686)
Allgemeine Beschreibung
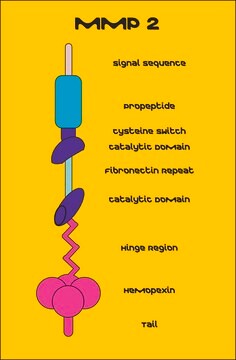
Matrix Metalloproteinase-2 (MMP-2) also known as gelatinase or type IV collagenase is a 72kDa protein. MMP-2 is a member of matrix metalloproteinase (MMP) family of enzymes. Basic structure of MMP2 contains signal peptide domain that targets the enzyme for secretion, the pro-peptide domain, which is removed when the enzyme is activated and the catalytic site containing gelatin-binding domain.
Spezifität
The amino acid sequences 1-662 of the proenzymes of MMP-2 are identical between mouse and rat.
Anwendung
Matrix metalloproteinase-2 (MMP2) human has been used as a standard in zymography to measure proteolytic activity of MMP-2.
Biochem./physiol. Wirkung
MMP-2 degrades general matrix components and may have a role in processes such as host defense, cell proliferation, and protein turnover as well as tissue remodeling.
Matrix Metalloproteinase-2 (MMP-2) cleaves gelatin, type IV, V, VII, X, and XI collagens, fibronectin, elastin, laminin, proteoglycans and a range of non extracellular matrix (ECM ) components. MMP-2 cleaves native type I collagen to N-terminal ¾ and C-terminal ¼ fragments identical to those generated by interstitial collagenases. MMP2 and MMP9 play an essential role in matrix degradation and they are implicated in the maintenance of neovascularization. In mice, deletion or inhibition of MMP2 protects against myocardial rupture.
Physikalische Form
Supplied as a 0.2 μm filtered solution of 25 mM Tris, pH 7.5, 5 mM calcium chloride, 75 mM sodium chloride, 0.025% Brij® 35 and 50% glycerol.
Hinweis zur Analyse
The biological activity is measured by its ability to cleave a fluorogenic peptide sustrate.
Rechtliche Hinweise
Brij is a registered trademark of Croda International PLC
Lagerklassenschlüssel
12 - Non Combustible Liquids
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Gelatinase A
Murphy, G. et al.
Handbook of Proteolytic Enzymes, 497-503 (2004)
INHIBITION OF GELATINASE ACTIVITY OF
MMP-2 AND MMP-9 BY EXTRACTS OF Bauhinia
ungulata L. Kamilla.
MMP-2 AND MMP-9 BY EXTRACTS OF Bauhinia
ungulata L. Kamilla.
Bioscience Journal, 31, 584-590 (2015)
Inhibition of Gelatinases by Vegetable Extracts
of the Species Tapirira guianensis
(Stick Pigeon).
of the Species Tapirira guianensis
(Stick Pigeon).
Longatti T R
British Journal of Pharmaceutical Research, 1(4), 133-140 (2011)
Immunohistochemical expression of MMP-14 and MMP-2, and MMP-2 activity during human ovarian follicular development.
Vos MC
Reproductive Biology and Endocrinology, 12:12 (2014)
P Reponen et al.
The Journal of biological chemistry, 267(11), 7856-7862 (1992-04-15)
We report the isolation of a cDNA clone providing the first and complete sequence of mouse 72-kDa type IV collagenase. The clone contains 2800 nucleotides with a 1986-nucleotide open reading frame coding for 662 amino acids. The amino acid sequence
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.