Alle Fotos(2)
Wichtige Dokumente
H5002
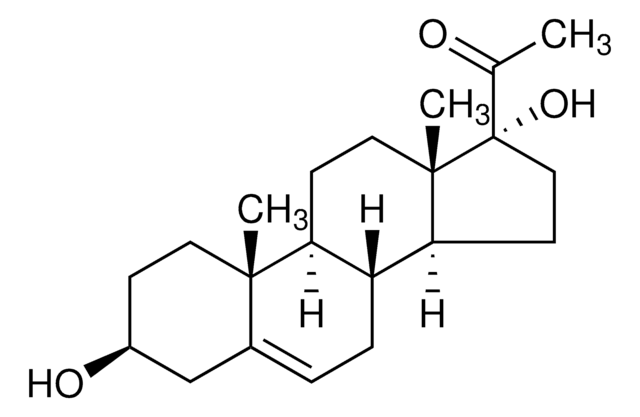
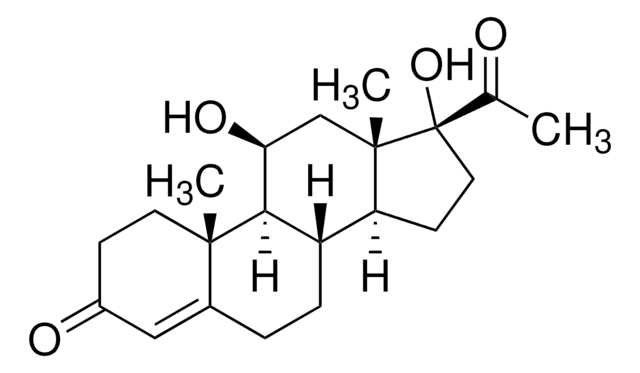
17α-Hydroxypregnenolone
Synonym(e):
3β,17α-Dihydroxy-5-pregnen-20-one, 5-Pregnene-3β,17α-diol-20-one
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C21H32O3
CAS-Nummer:
Molekulargewicht:
332.48
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
41116107
PubChem Substanz-ID:
NACRES:
NA.77
Empfohlene Produkte
Qualitätsniveau
SMILES String
C[C@]1(CC[C@H](O)C2)C2=CC[C@]3([H])[C@]1([H])CC[C@@]4(C)[C@@]3([H])CC[C@@]4(C(C)=O)O
InChI
1S/C21H32O3/c1-13(22)21(24)11-8-18-16-5-4-14-12-15(23)6-9-19(14,2)17(16)7-10-20(18,21)3/h4,15-18,23-24H,5-12H2,1-3H3
InChIKey
JERGUCIJOXJXHF-UHFFFAOYSA-N
Allgemeine Beschreibung
17α-hydroxypregnenolone is a derived from pregnenolone.
Anwendung
17α-hydroxypregnenolone has been used as a substrate for the enzyme 3β‐hydroxysteroid dehydrogenase (3β‐HSD) expressed in COS1 cells.
Biochem./physiol. Wirkung
17α-hydroxypregnenolone acts as a precursor for cortisol and sex steroids.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Shogo Haraguchi et al.
Endocrinology, 153(2), 794-805 (2011-12-01)
7α-Hydroxypregnenolone (7α-OH PREG) is a newly identified bioactive neurosteroid stimulating locomotor activity in the brain of newt, a wild animal, which serves as an excellent model to investigate the biosynthesis and biological action of neurosteroids. Here, we show that acute
Kazuyoshi Tsutsui et al.
General and comparative endocrinology, 168(2), 275-279 (2010-02-09)
We now know that steroids can be synthesized de novo by the brain and the peripheral nervous system. Such steroids are called neurosteroids and de novo neurosteroidogenesis from cholesterol is a conserved property of vertebrate brains. Our studies over the
Kazuyoshi Tsutsui et al.
General and comparative endocrinology, 176(3), 440-447 (2011-12-06)
Seasonally-breeding amphibians have served as excellent animal models to investigate the biosynthesis and biological actions of neurosteroids. Previous studies have demonstrated that the brain of amphibians possesses key steroidogenic enzymes and produces pregnenolone, a precursor of steroid hormones, and other
Tamara S Hannon et al.
Journal of pediatric and adolescent gynecology, 25(1), 82-85 (2011-11-18)
Little is known about racial differences in androgen levels among obese children. The objective of this pilot study was to compare basal and stimulated androgen levels in a cross-sectional sample of obese black and white pubertal females. STUDY DESIGN, SETTING
Teppei Koyama et al.
Annals of the New York Academy of Sciences, 1163, 444-447 (2009-05-22)
We recently identified 7alpha-hydroxypregnenolone as a novel amphibian neurosteroid stimulating locomotor activity in newts. Because male newts show marked diurnal changes in locomotor activity, we hypothesized that 7alpha-hydroxypregnenolone may be a key factor for the induction of diurnal changes in
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.