Alle Fotos(1)
Wichtige Dokumente
D7408
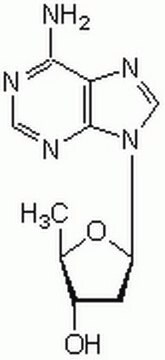
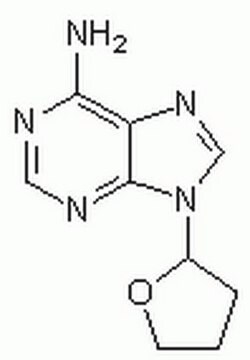
2′,5′-Dideoxyadenosine
≥95% (HPLC), solid
Synonym(e):
2ʹ,5ʹ-dd-Ado, NSC 95943
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C10H13N5O2
CAS-Nummer:
Molekulargewicht:
235.24
MDL-Nummer:
UNSPSC-Code:
41106305
PubChem Substanz-ID:
NACRES:
NA.77
Empfohlene Produkte
Assay
≥95% (HPLC)
Form
solid
Farbe
white
Löslichkeit
DMSO: soluble
Lagertemp.
−20°C
SMILES String
C[C@H]1O[C@H](C[C@@H]1O)n2cnc3c(N)ncnc23
InChI
1S/C10H13N5O2/c1-5-6(16)2-7(17-5)15-4-14-8-9(11)12-3-13-10(8)15/h3-7,16H,2H2,1H3,(H2,11,12,13)/t5-,6+,7-/m1/s1
InChIKey
FFHPXOJTVQDVMO-DSYKOEDSSA-N
Angaben zum Gen
rat ... Adcy2(81636)
Anwendung
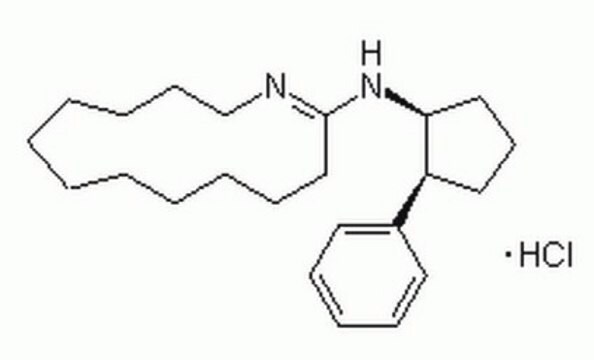
2′,5′-Dideoxyadenosine has been used to elucidate the mechanism of diligustilide (DLG). It has also been used to inhibit adenylate cyclase (AC).
Biochem./physiol. Wirkung
Cell-permeable adenylyl cyclase inhibitor. IC50 = 2.7 μM in detergent-dispersed rat brain preparations.
Leistungsmerkmale und Vorteile
This compound is a featured product for Cyclic Nucleotide research. Click here to discover more featured Cyclic Nucleotide products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the Adenylyl cyclases page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Rekonstituierung
Store at −20 °C after reconstitution.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Rula Azzam et al.
AIDS research and human retroviruses, 22(7), 619-629 (2006-07-13)
HIV-1 infection of cells of macrophage lineage impairs a number of effector functions performed by these cells, including phagocytosis of opsonized pathogens. In this study we investigate the effects of HIV-1 on the mechanism of complement (C')-mediated phagocytosis by human
Gastroprotective effect of diligustilide isolated from roots of Ligusticum porteri coulter & rose (Apiaceae) on ethanol-induced lesions in rats
Velazquez-Moyado J, et al.
Journal of Ethnopharmacology, 174, 403-409 (2015)
Yukihisa Matsumoto et al.
PloS one, 8(7), e68538-e68538 (2013-07-31)
Many insects exhibit excellent capability of visual learning, but the molecular and neural mechanisms are poorly understood. This is in contrast to accumulation of information on molecular and neural mechanisms of olfactory learning in insects. In olfactory learning in insects
Fen Hu et al.
Molecular medicine reports, 24(4) (2021-07-31)
Inflammation and oxidative stress have indispensable roles in the development of acute lung injury (ALI). MicroRNA (miRNA/miR)‑351‑5p was initially identified as a myogenesis‑associated miRNA; however, its role in lipopolysaccharide (LPS)‑induced ALI remains unclear. The aim of the present study was
Hyun-Seuk Moon et al.
Journal of cellular physiology, 214(2), 283-294 (2007-07-27)
We previously reported that PEGylated conjugated linoleic acid (PCLA) as a pro-drug treatment of cultures of 3T3-L1 cells containing differentiated adipocytes caused de-differentiation by downregulation of PPARgamma2-induced adipogenesis, and cell apoptosis induced by PCLA was lower than that induced by
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.