Alle Fotos(1)
Wichtige Dokumente
A0912
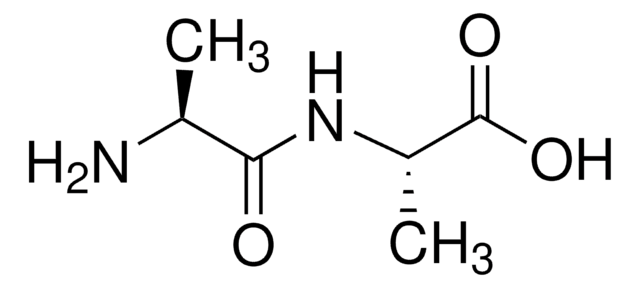
D-Ala-D-Ala
≥99%
Synonym(e):
D-alanyl-D-Alanine
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C6H12N2O3
CAS-Nummer:
Molekulargewicht:
160.17
MDL-Nummer:
UNSPSC-Code:
12352202
PubChem Substanz-ID:
NACRES:
NA.26
Empfohlene Produkte
Produktbezeichnung
D-Ala-D-Ala,
Assay
≥99%
Form
powder
Farbe
white to off-white
Lagertemp.
−20°C
SMILES String
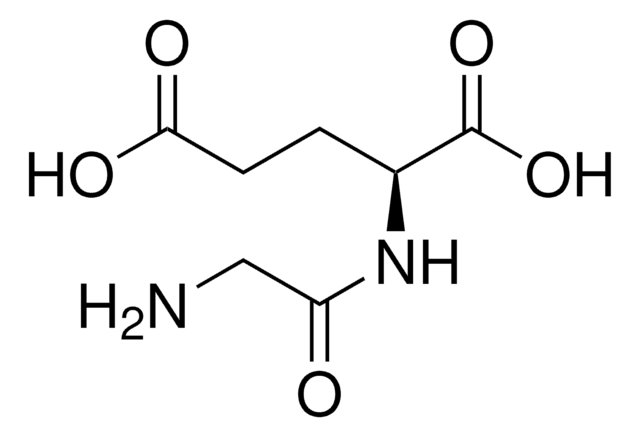
C[C@@H](N)C(=O)N[C@H](C)C(O)=O
InChI
1S/C6H12N2O3/c1-3(7)5(9)8-4(2)6(10)11/h3-4H,7H2,1-2H3,(H,8,9)(H,10,11)/t3-,4-/m1/s1
InChIKey
DEFJQIDDEAULHB-QWWZWVQMSA-N
Anwendung
- Binding Mode-Based Physicochemical Screening Method Using d-Ala-d-Ala Silica Gel and Chemical Modification Approach to Facilitate Discovery of New Macrolactams, Banglactams A and B, from Nonomuraea bangladeshensis K18-0086.: Describes a novel screening method employing D-Ala-D-Ala silica gel to discover new macrolactams with potential antibacterial properties. This technique aids in identifying compounds that inhibit bacterial cell wall synthesis (Kimishima et al., 2024).
Biochem./physiol. Wirkung
D-Ala-D-Ala is found in the stem termini of peptidoglycan side-chain pentapeptide found in the cell walls of gram positive bacteria. The D-ala-d-ala stem termini is the site of interaction of glycopeptide antibiotics such as vancomycin and teicoplanin. D-ala-D-ala is a substrate used to study kinetics of UDPMurNAc-tripeptide D-alanyl-D-alanine-adding (ligase) enzyme.
D-Ala-D-Ala, a terminus moiety of bacterial peptidoglycans, is used for affinity chromatography and binding mechanism studies of antibiotics such as teicoplanin, ristocetin, vancomycin.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Yoshiaki Kitamura et al.
Acta crystallographica. Section D, Biological crystallography, 65(Pt 10), 1098-1106 (2009-09-23)
D-Alanine-D-alanine ligase (Ddl) is one of the key enzymes in peptidoglycan biosynthesis and is an important target for drug discovery. The enzyme catalyzes the condensation of two D-Ala molecules using ATP to produce D-Ala-D-Ala, which is the terminal peptide of
Maulik N Thaker et al.
Antimicrobial agents and chemotherapy, 59(3), 1405-1410 (2014-12-17)
Vancomycin-resistant enterococci (VRE) are notorious clinical pathogens restricting the use of glycopeptide antibiotics in the clinic setting. Routine surveillance to detect VRE isolated from patients relies on PCR bioassays and chromogenic agar-based test methods. In recent years, we and others
Gareth A Prosser et al.
Antimicrobial agents and chemotherapy, 60(10), 6091-6099 (2016-08-03)
The increasing global prevalence of drug resistance among many leading human pathogens necessitates both the development of antibiotics with novel mechanisms of action and a better understanding of the physiological activities of preexisting clinically effective drugs. Inhibition of peptidoglycan (PG)
I Tytgat et al.
Current medicinal chemistry, 16(20), 2566-2580 (2009-07-16)
DD-ligases catalyze the synthesis of the D-Ala-D-Ala and D-Ala-D-Ser dipeptides or the D Ala-D-Lac depsipeptide in an early step of peptidoglycan synthesis. Their function is essential for bacterial growth and specific to bacteria, making them attractive targets for the development
Ivona Pavkova et al.
Frontiers in cellular and infection microbiology, 7, 503-503 (2018-01-13)
The DsbA homolog of
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.