Wichtige Dokumente
38132
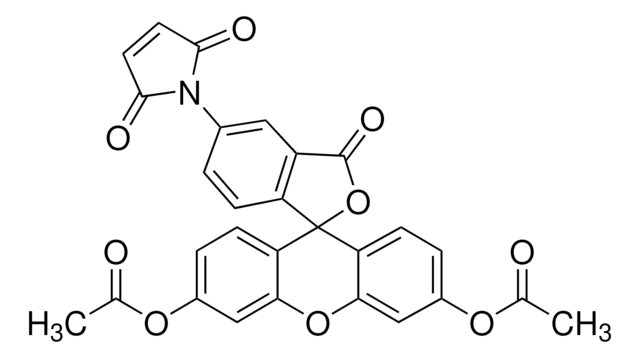
N-(5-Fluoreszeinyl)maleimid
≥90% (HPLC), BioReagent, suitable for fluorescence
Synonym(e):
5-Maleinimido-fluorescein
About This Item
Empfohlene Produkte
Produktlinie
BioReagent
Qualitätsniveau
Assay
≥90% (HPLC)
Fluoreszenz
λex 490 nm; λem 518 nm in 0.1 M Tris pH 8.0
Eignung
corresponds for coupling to thiols
suitable for fluorescence
Lagertemp.
2-8°C
SMILES String
Oc1ccc2c(Oc3cc(O)ccc3C24OC(=O)c5cc(ccc45)N6C(=O)C=CC6=O)c1
InChI
1S/C24H13NO7/c26-13-2-5-17-19(10-13)31-20-11-14(27)3-6-18(20)24(17)16-4-1-12(9-15(16)23(30)32-24)25-21(28)7-8-22(25)29/h1-11,26-27H
InChIKey
AYDAHOIUHVUJHQ-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
N-(5-Fluoresceinyl) maleimide (5-FM) is used to fluorescence label molecules such as proteins and peptides via their thiol groups.
Verpackung
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.