77634
Kalibriersubstanz von Mettler Toledo, ME 51143093, Vanillin
traceable to primary standards (LGC)
Synonym(e):
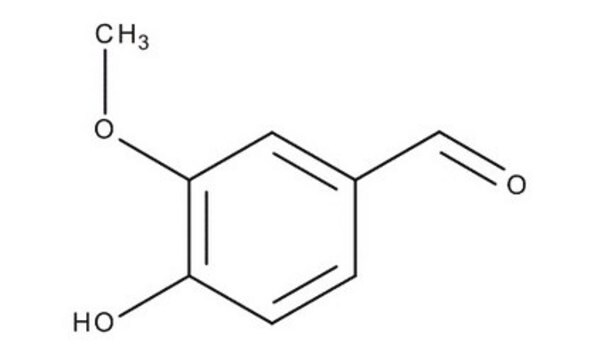
Vanillin, 4-Hydroxy-3-methoxybenzaldehyd
About This Item
Empfohlene Produkte
Qualität
analytical standard
traceable to primary standards (LGC)
Qualitätsniveau
Dampfdichte
5.3 (vs air)
Dampfdruck
>0.01 mmHg ( 25 °C)
Haltbarkeit
limited shelf life, expiry date on the label
bp
170 °C/15 mmHg (lit.)
mp (Schmelzpunkt)
81-83 °C (lit.)
Anwendung(en)
food and beverages
pharmaceutical
Format
neat
SMILES String
COc1cc(C=O)ccc1O
InChI
1S/C8H8O3/c1-11-8-4-6(5-9)2-3-7(8)10/h2-5,10H,1H3
InChIKey
MWOOGOJBHIARFG-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Mettler-Toledo calibration substance ME 51143093, vanillin is an analytical standard for use in the regular checking of Mettler-Toledo melting point instrument. Its value equals an average of 6 to 12 measurements with a Mettler-Toledo MP90 Excellence instrument that is calibrated against primary standards. The melting point is validated by Capillary method according to European Pharmacopeia (2.2.14.)
Anwendung
Leistungsmerkmale und Vorteile
- Traceable to a primary standard (LGC, London)
- Melting point evaluation conducted in both thermodynamic and pharmacopeia modes for physically correct and heating rate dependent melting point determinations, respectively
- Provided with certificates of analysis and safety data sheet
- A product of analytical standard grade to help meet the QC/QA requirements of melting point determination
- Standard deviation up to ± 0.2 °C
Empfohlene Produkte
Rechtliche Hinweise
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 1
Flammpunkt (°F)
319.6 - 321.4 °F - closed cup
Flammpunkt (°C)
159.8 - 160.8 °C - closed cup
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.