26454
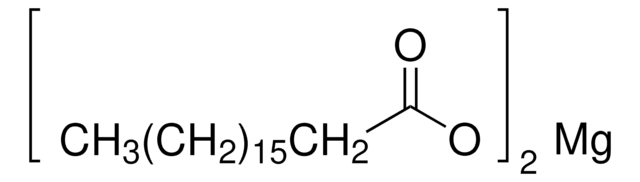
Magnesiumstearat
puriss., meets analytical specification of Ph. Eur., BP, ≥90% stearic and palmitic acid basis, ≥40% stearic acid basis (GC), 4.0-5.0% Mg basis (calc on dry sub.)
Synonym(e):
Stearinsäure Magnesiumsalz
About This Item
Empfohlene Produkte
Qualität
puriss.
meets analytical specification of Ph. Eur., BP
Assay
≥40% stearic acid basis (GC)
≥90% stearic and palmitic acid basis
4.0-5.0% Mg basis (calc on dry sub.)
Verunreinigungen
acidity or alkalinity, complies
microbiological impurity, in accordance
residual solvents, complies
Verlust
≤6.0% loss on drying, 105 °C
mp (Schmelzpunkt)
200 °C (lit.)
Säurezahl
195‑210(fatty acid)
Löslichkeit
alcohol: insoluble
diethyl ether: insoluble
water: insoluble
Anionenspuren
chloride (Cl-): ≤1000 mg/kg
sulfate (SO42-): ≤5000 mg/kg
Kationenspuren
Cd: ≤3 mg/kg
Ni: ≤5 mg/kg
Pb: ≤10 mg/kg
Eignung
in accordance for fatty acid composition
passes test for identity
Anwendung(en)
pharmaceutical (small molecule)
SMILES String
CCCCCCCCCCCCCCCCCC(=O)O[Mg]OC(=O)CCCCCCCCCCCCCCCCC
InChI
1S/2C18H36O2.Mg/c2*1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20;/h2*2-17H2,1H3,(H,19,20);/q;;+2/p-2
InChIKey
HQKMJHAJHXVSDF-UHFFFAOYSA-L
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
Biochem./physiol. Wirkung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
nwg
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.