800100O
Avanti
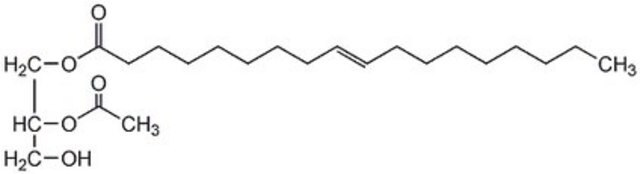
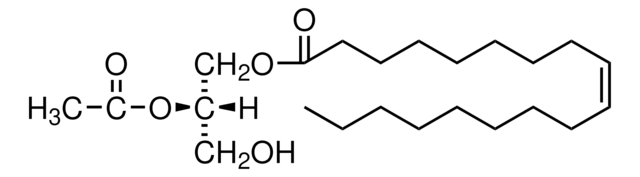
18:1-2:0 DG
1-oleoyl-2-acetyl-sn-glycerol, neat oil
Synonym(e):
1--(9Z-octadecenoyl)-2-acetoyl-sn-glycerol; DG(18:1(9Z)/2:0/0:0); OAG
About This Item
Empfohlene Produkte
Assay
>99% (TLC)
Form
liquid
Verpackung
pkg of 1 × 10 mg (with screw cap (800100O-10mg))
pkg of 1 × 25 mg (with screw cap (800100O-25mg))
Hersteller/Markenname
Avanti Research™ - A Croda Brand 800100O
Lipid-Typ
neutral glycerides
neutral lipids
Versandbedingung
dry ice
Lagertemp.
−20°C
SMILES String
O([C@H](COC(=O)CCCCCCC\C=C/CCCCCCCC)CO)C(=O)C
InChI
1S/C23H42O5/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-23(26)27-20-22(19-24)28-21(2)25/h10-11,22,24H,3-9,12-20H2,1-2H3/b11-10-/t22-/m0/s1
InChIKey
PWTCCMJTPHCGMS-YRBAHSOBSA-N
Allgemeine Beschreibung
Anwendung
- in the induction of superoxide generation in neutrophils
- as a substrate for platelet-activating factor acetyl hydrolases
- as a protein kinase C (PKC) activator in adrenal glands
Biochem./physiol. Wirkung
Verpackung
Lagerung und Haltbarkeit
Sonstige Hinweise
Rechtliche Hinweise
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumente section.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.