W249311
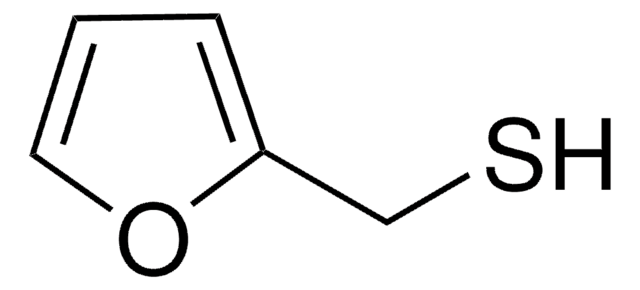
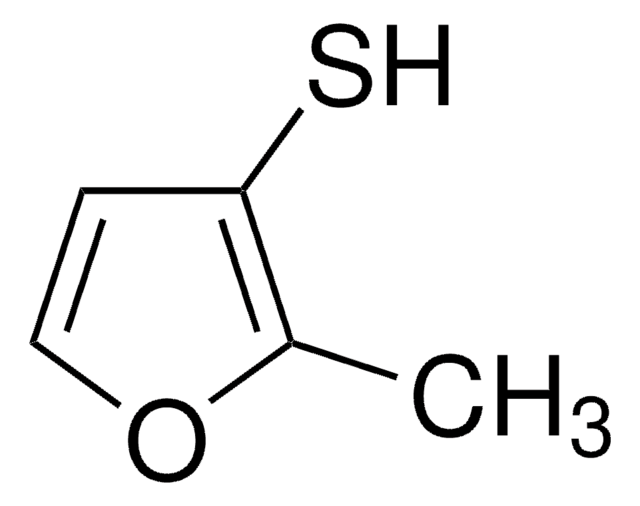
2-Furanmethanthiol
natural, 98%, FG
Synonym(e):
2-Furfurylthiol, 2-Furylmethanthiol, Furfurylmercaptan
About This Item
Empfohlene Produkte
Qualität
FG
Fragrance grade
Halal
Kosher
natural
Agentur
follows IFRA guidelines
meets purity specifications of JECFA
Einhaltung gesetzlicher Vorschriften
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
Assay
98%
Grünere Alternativprodukt-Eigenschaften
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Brechungsindex
n20/D 1.531 (lit.)
bp
155 °C (lit.)
Dichte
1.132 g/mL at 25 °C (lit.)
Anwendung(en)
flavors and fragrances
Dokumentation
see Safety & Documentation for available documents
Nahrungsmittelallergen
no known allergens
Allergener Duftstoff
no known allergens
Grünere Alternativprodukt-Kategorie
Organoleptisch
coffee; meaty; roasted; sulfurous
SMILES String
SCc1ccco1
InChI
1S/C5H6OS/c7-4-5-2-1-3-6-5/h1-3,7H,4H2
InChIKey
ZFFTZDQKIXPDAF-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Sonstige Hinweise
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.