F6129
Eisencitrat
technical grade
Synonym(e):
Eisen(III)-citrat, Eisen(III)-citrat-Hydrat
About This Item
Empfohlene Produkte
Qualität
technical grade
Form
powder
Zusammensetzung
Fe, 16.5-18.5%
Methode(n)
cell culture | mammalian: suitable
Anwendung(en)
battery manufacturing
SMILES String
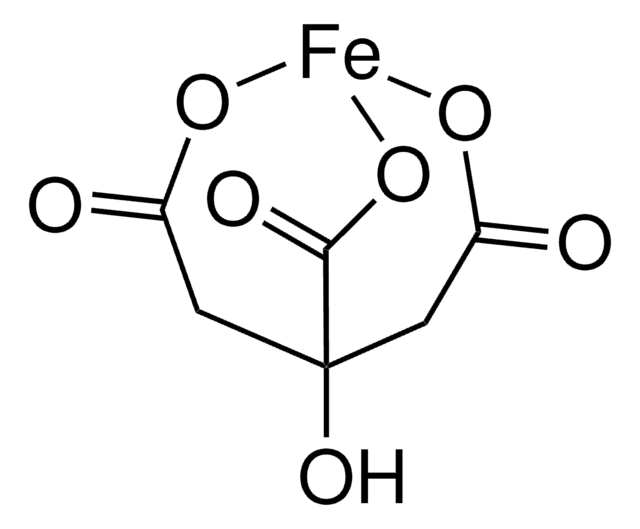
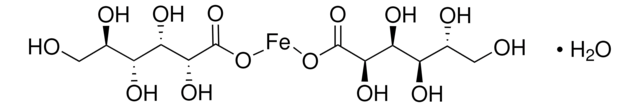
OC12CC(=O)O[Fe](OC(=O)C1)OC2=O
InChI
1S/C6H8O7.Fe/c7-3(8)1-6(13,5(11)12)2-4(9)10;/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12);/q;+3/p-3
InChIKey
NPFOYSMITVOQOS-UHFFFAOYSA-K
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
It can also be used in the degradation of tetracycline for the treatment of polluted water.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Plasmonic nanoparticles have unique optical properties that can be tailored to suit a variety of applications in the biotechnology1–8 and electronics9–16 industries.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.