Alle Fotos(1)
Wichtige Dokumente
D87589
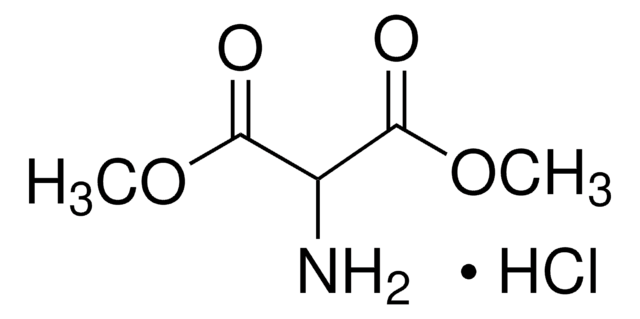
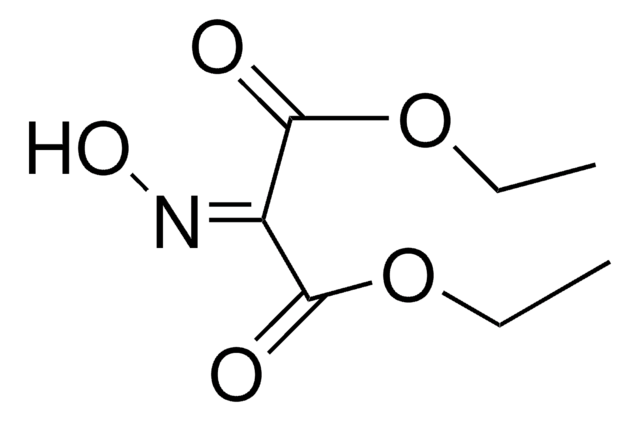
Aminomalonsäure-diethylester -hydrochlorid
98%
Synonym(e):
Diethyl-aminomalonat -hydrochlorid
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
NH2CH2(COOC2H5)2 · HCl
CAS-Nummer:
Molekulargewicht:
211.64
Beilstein:
3568037
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Form
crystals
mp (Schmelzpunkt)
165-170 °C (dec.) (lit.)
SMILES String
Cl.CCOC(=O)C(N)C(=O)OCC
InChI
1S/C7H13NO4.ClH/c1-3-11-6(9)5(8)7(10)12-4-2;/h5H,3-4,8H2,1-2H3;1H
InChIKey
GLFVNTDRBTZJIY-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
C M Metzler et al.
Biochemistry, 27(13), 4923-4933 (1988-06-28)
To establish the state of protonation of quinonoid species formed nonenzymically from pyridoxal phosphate (PLP) and diethyl aminomalonate, we have studied absorption spectra of the rapidly established steady-state mixture of species. We have evaluated the formation constant and the spectrum
Seiichi Ohta et al.
Molecular pharmaceutics, 14(9), 3105-3113 (2017-08-15)
Intraperitoneal administration of chemotherapeutics is expected for the treatment of peritoneally disseminated gastric cancer because of poor migration of the drugs from the systemic circulation to the peritoneal cavity. In this study, for intraperitoneal delivery of cisplatin (CDDP), we developed
Reaction control in the organocatalytic asymmetric one-pot, three-component reaction of aldehydes, diethyl alpha-aminomalonate and nitroalkenes: toward diversity-oriented synthesis.
Yan-Kai Liu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(32), 9873-9877 (2008-10-04)
F Hughes et al.
Organic letters, 3(18), 2911-2914 (2001-09-01)
[reaction: see text]. Nitrogen-containing tethered diacids, easily prepared by reductive alkylation of diethyl aminomalonate or ethyl cyanoglycinate, undergo double Michael reactions with 3-butyn-2-one to give highly functionalized and substituted piperidines (pipecolic acid derivatives) with surprisingly high stereoselectivity. The heterocyclic double
Charles M Blazey et al.
The Journal of organic chemistry, 67(1), 298-300 (2002-01-05)
The azomethine ylide derived from the condensation of diethyl aminomalonate with paraformaldehyde undergoes 1,3-dipolar cycloadditions with acrylate and propiolate derivatives. Contrary to a previous report, these reactions yield mixtures of regioisomers generally favoring the 2,2,3-trisubstituted product. However, the relative quantity
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.