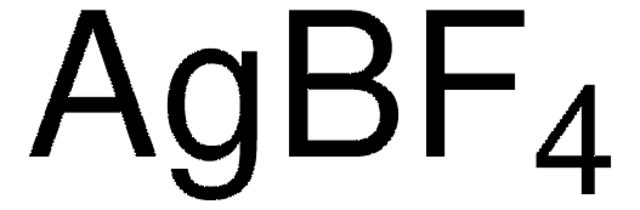Wichtige Dokumente
674583
Silberperchlorat
anhydrous, 97%
Synonym(e):
Perchloric acid silver(1+) salt, Silver(1+) perchlorate
About This Item
Empfohlene Produkte
Qualität
anhydrous
Qualitätsniveau
Assay
97%
Form
solid
Eignung der Reaktion
reagent type: catalyst
core: silver
Verunreinigungen
≤0.1% Insoluble matter
mp (Schmelzpunkt)
486 °C (lit.)
Löslichkeit
benzene: slightly soluble(lit.)
pyridine: slightly soluble(lit.)
Dichte
2.8 g/mL at 25 °C (lit.)
SMILES String
[Ag+].[O-]Cl(=O)(=O)=O
InChI
1S/Ag.ClHO4/c;2-1(3,4)5/h;(H,2,3,4,5)/q+1;/p-1
InChIKey
YDHABVNRCBNRNZ-UHFFFAOYSA-M
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
- Anion exchange dynamics in the capture of perchlorate by a cationic Ag-based MOF: Investigates the anion exchange dynamics and structural transformations of a silver-based metal-organic framework capturing perchlorate (Colinas et al., 2017).
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Ox. Sol. 2 - Skin Corr. 1B
Lagerklassenschlüssel
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
We are proud to offer a treasure-trove of gold precatalysts and silver salts, as well as an extensive portfolio of unsaturated building blocks to accelerate your research success in this exciting field.
Plasmonic nanoparticles have unique optical properties that can be tailored to suit a variety of applications in the biotechnology1–8 and electronics9–16 industries.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.