Alle Fotos(1)
Wichtige Dokumente
524212
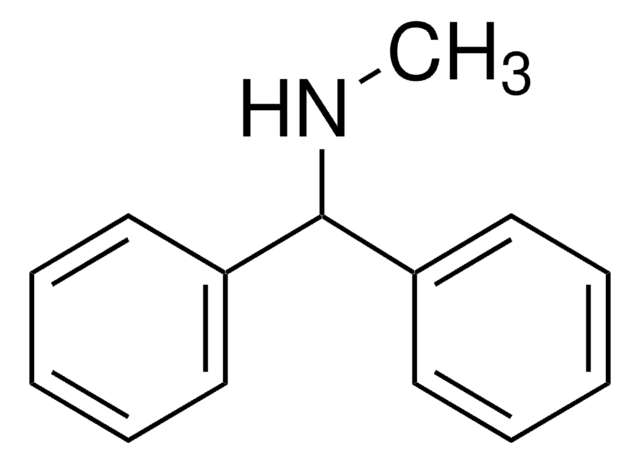
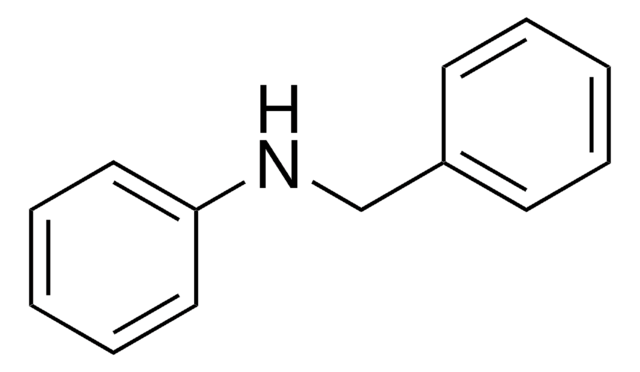
N-Methyldiphenylamin
96%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
(C6H5)2NCH3
CAS-Nummer:
Molekulargewicht:
183.25
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
96%
Brechungsindex
n20/D 1.623 (lit.)
bp
293 °C (lit.)
Dichte
1.05 g/mL at 25 °C (lit.)
Funktionelle Gruppe
amine
SMILES String
CN(c1ccccc1)c2ccccc2
InChI
1S/C13H13N/c1-14(12-8-4-2-5-9-12)13-10-6-3-7-11-13/h2-11H,1H3
InChIKey
DYFFAVRFJWYYQO-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
N-Methyldiphenylamine is an aromatic tertiary amine. It undergoes transformation to N-methylcarbazole (C) via a photochemical reaction.
Anwendung
N-Methyldiphenylamine may be used for the synthesis of phosphonium ion salts. It may also be used as a starting reagent for the preparation of bis(4-carboxyphenyl)-N-methylamine (H2CPMA).
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
230.0 °F - closed cup
Flammpunkt (°C)
110 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Tae Kyung Kim et al.
Inorganic chemistry, 52(2), 589-595 (2012-12-29)
A luminescent lithium metal-organic framework (MOF) is constructed from the solvothermal reaction of Li(+) and a well-designed organic ligand, bis(4-carboxyphenyl)-N-methylamine (H(2)CPMA). A Li-based MOF can detect an explosive aromatic compound containing nitro groups as an explosophore, by showing a dramatic
Reaction patterns and kinetics of the photoconversion of N-methyldiphenylamine to N-methylcarbazole.
Forster EW, et al.
Journal of the American Chemical Society, 95(10), 3108-3115 (1973)
Frustrated Lewis pair-mediated C?O or C?H bond activation of ethers.
Holthausen MH, et al.
Chemical Communications (Cambridge, England), 50(70), 10038-10040 (2014)
Natália M Monezi et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 173, 462-467 (2016-10-08)
The distinct thermochromism observed in solutions containing N,N-dimethylaniline (DMA) and N,N-diethylaniline (DEA) and SO2 was investigated by resonance Raman spectroscopy in a wide range of temperatures. The results indicate in addition to the charge transfer (CT) complexes DMA-SO2 and DEA-SO2
Minoru Yamaji et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 14(9), 1673-1684 (2015-07-07)
Photochemical processes of 4-tert-butyl-4'-methoxydibenzoylmethane (Avobenzone, AB), 4-phenylbenzoylbenzoyl-, 4-phenylbenzoyl-2'-furanyl- and 4-phenylbenzoyl-2'-thenoylmethanes (PB@Ph, PB@F and PB@T, respectively) substituted with Br and Cl at the C2 position were studied by stationary and laser flash photolyses in solution. The absorption spectral features showed that
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.