512125
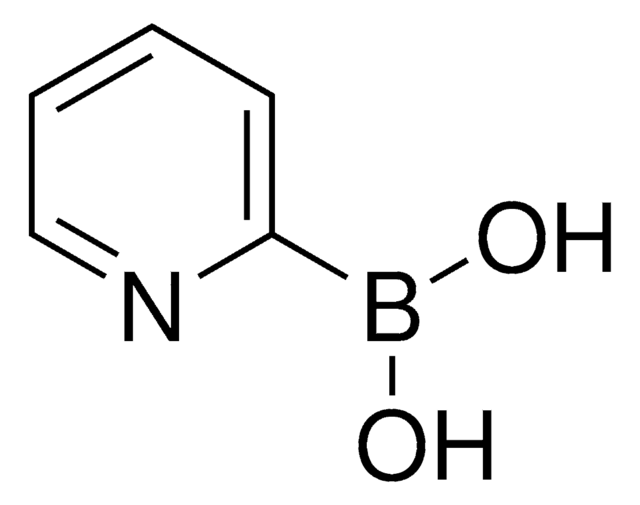
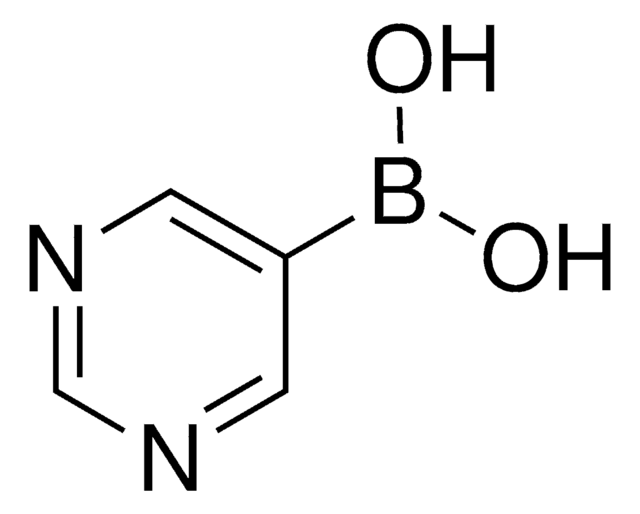
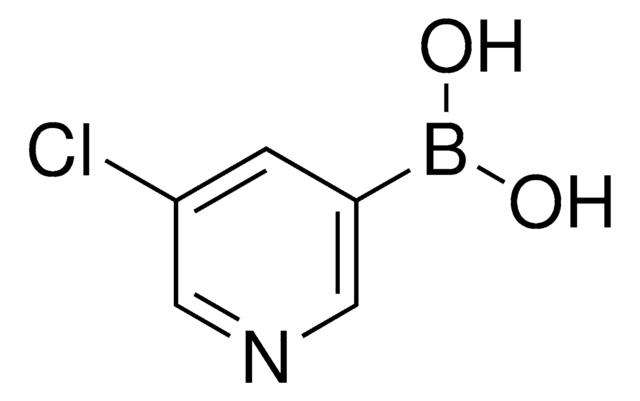
3-Pyridinboronsäure
≥95.0%
Synonym(e):
3-Pyridinylborsäure
About This Item
Empfohlene Produkte
Assay
≥95.0%
Form
solid
mp (Schmelzpunkt)
>300 °C (lit.)
SMILES String
OB(O)c1cccnc1
InChI
1S/C5H6BNO2/c8-6(9)5-2-1-3-7-4-5/h1-4,8-9H
InChIKey
ABMYEXAYWZJVOV-UHFFFAOYSA-N
Verwandte Kategorien
Anwendung
- Phosphine-free Suzuki-Miyaura cross-coupling reactions.
- Regioselective Suzuki-Miyaura coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulation.
- N-arylation using copper acetylacetonate catalyst.
- Copper-mediated cyanation and regioselective cyanation of electron-rich benzenes.
It can also be used to prepare:
- New linear poly(phenylpyridyl) chains by Suzuki coupling.
- Oligopyridyl foldamers as mimics of a-helix twist.
- Many highly significant therapeutic enzymatic and kinase inhibitors and receptor antagonists.
- Pyridine substituted pyridinium N-(2′-azinyl)aminides by reacting with dibromo pyridinium aminides via Suzuki coupling reaction.
Sonstige Hinweise
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.