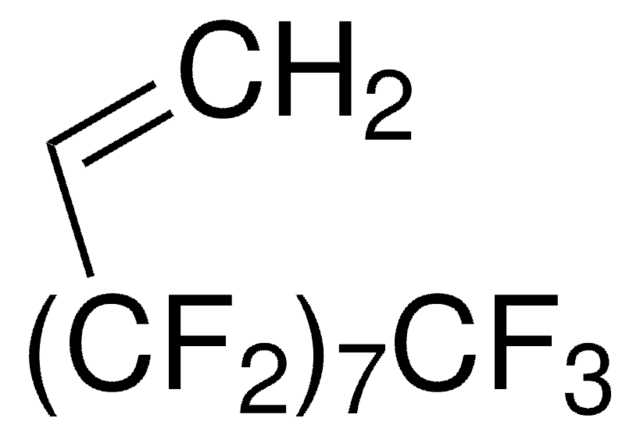Wichtige Dokumente
474223
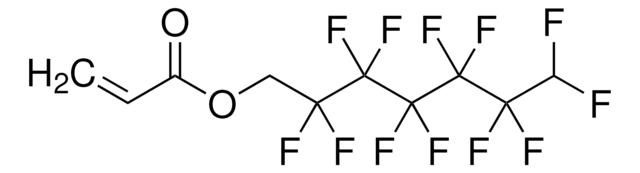
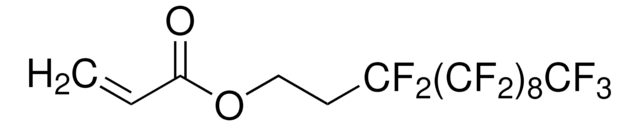
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-Heptadecafluordecylmethacrylat
contains MEHQ as inhibitor, 97%
Synonym(e):
HDFDMA
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Enthält
MEHQ as inhibitor
Brechungsindex
n20/D 1.343 (lit.)
bp
110 °C/4 mmHg (lit.)
Dichte
1.596 g/mL at 25 °C (lit.)
Lagertemp.
2-8°C
SMILES String
CC(=C)C(=O)OCCC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F
InChI
1S/C14H9F17O2/c1-5(2)6(32)33-4-3-7(15,16)8(17,18)9(19,20)10(21,22)11(23,24)12(25,26)13(27,28)14(29,30)31/h1,3-4H2,2H3
InChIKey
HBZFBSFGXQBQTB-UHFFFAOYSA-N
Allgemeine Beschreibung
Anwendung
- As a monomer to synthesize poly(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-Heptadecafluorodecyl methacrylate) (PFDMA). PFDMA can be used to prepare a super amphiphobic sponge that repels both water and oil via the initiated chemical vapor deposition(iCVD).
- As a surface modification agent to alter the surface energy of steel-use-stainless (SUS) membrane to separate microalgal lipids from wet biomass. This helps to simplify the process of microalgae-derived biodiesel production.
- To fabricate biocompatible polymer coatings for transmucosal delivery of oral drugs. The polymer coating induces hydrophobicity that helps to avoid the release of drugs in unwanted directions.
- As a precursor/monomer to prepare triblock copolymers as electrolytes for Li metal batteries. It enhances mechanical strength, lithium-ion transference number, and cycling stability.
Signalwort
Danger
Gefahreneinstufungen
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Lact. - Repr. 1B - STOT RE 1
Zielorgane
Liver
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flammpunkt (°F)
235.4 °F - closed cup
Flammpunkt (°C)
113 °C - closed cup
Persönliche Schutzausrüstung
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.