Wichtige Dokumente
434191
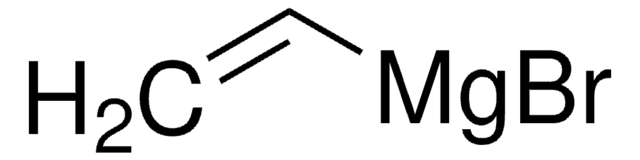
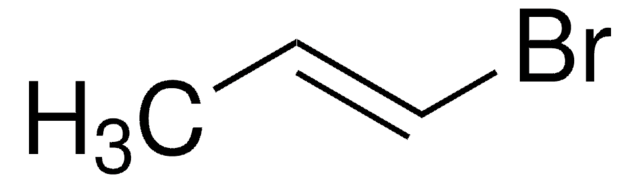
Vinylbromid -Lösung
1.0 M in THF
Synonym(e):
Bromethylen
About This Item
Empfohlene Produkte
Dampfdruck
12.46 psi ( 55 °C)
3.6 psi ( 20 °C)
Qualitätsniveau
Form
liquid
Konzentration
1.0 M in THF
Dichte
0.927 g/mL at 25 °C
Lagertemp.
2-8°C
SMILES String
BrC=C
InChI
1S/C2H3Br/c1-2-3/h2H,1H2
InChIKey
INLLPKCGLOXCIV-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- Sequential Vinyl Radical Cyclization/Fixation of Carbon Dioxide through Electrochemical Reduction of Vinyl Bromide in the Presence of an Electron-Transfer Mediator: This study explores the electrochemical reduction of vinyl bromide with a focus on vinyl radical cyclization and carbon dioxide fixation (A Katayama, H Senboku, 2016).
- A Comparison of the Wavelength-Dependent Photochemical Reactions of Ozone with Vinyl Bromide and Fluoride in Argon Matrices: The study compares the photochemical reactions of vinyl bromide and fluoride with ozone, examining their behavior in argon matrices (BS Ault, 2021).
Signalwort
Danger
Gefahreneinstufungen
Acute Tox. 4 Oral - Carc. 1B - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Zielorgane
Central nervous system, Respiratory system
Zusätzliche Gefahrenhinweise
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 3
Flammpunkt (°F)
1.4 °F - closed cup
Flammpunkt (°C)
-17 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.