Alle Fotos(3)
Wichtige Dokumente
433063
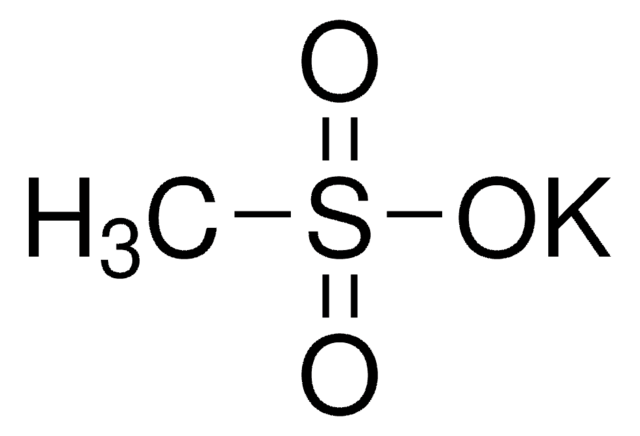
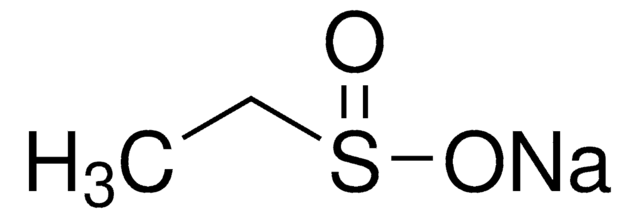
Natriummethansulfinat
technical grade, 85%
Synonym(e):
Methansulfinsäure Natriumsalz
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Lineare Formel:
CH3SO2Na
CAS-Nummer:
Molekulargewicht:
102.09
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualität
technical grade
Qualitätsniveau
Assay
85%
mp (Schmelzpunkt)
222-226 °C (dec.) (lit.)
Funktionelle Gruppe
sulfinic acid
SMILES String
[Na+].CS([O-])=O
InChI
1S/CH4O2S.Na/c1-4(2)3;/h1H3,(H,2,3);/q;+1/p-1
InChIKey
LYPGDCWPTHTUDO-UHFFFAOYSA-M
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Sodium methanesulfinate is an aliphatic sodium sulfinate. Conjugate addition of sodium methanesulfinate to vinyl heterocycles has been described. Cross-coupling reaction between aryl boronic acid and sodium methanesulfinate has been studied. Its stock solution was prepared from methanesulfonic acid by adding one equivalent of sodium hydroxide and diluting it to 4M.
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Metal-Free Direct Construction of Sulfonamides via Iodine-Mediated Coupling Reaction of Sodium Sulfinates and Amines at Room Temperature.
Wei W, et al.
Advanced Synthesis & Catalysis, 357(5), 987-992 (2015)
J B Smith et al.
Free radical research communications, 8(2), 101-106 (1990-01-01)
Iron bound to certain chelators is known to promote the conversion of superoxide radicals (O2-) to hydroxyl radicals (HO.) by the superoxide-driven Fenton reaction. The production of HO. by various iron chelates was studied using the reaction of dimethyl sulfoxide
M G Steiner et al.
Archives of biochemistry and biophysics, 278(2), 478-481 (1990-05-01)
This investigation was conducted to validate the use of dimethyl sulfoxide (DMSO) as a quantitative molecular probe for the generation of hydroxyl radicals (HO.) in aqueous systems. Reaction of HO. with DMSO produces methane sulfinic acid as a primary product
M G Steiner et al.
Free radical biology & medicine, 9(1), 67-77 (1990-01-01)
To quantitate the formation of hydroxyl radicals (HO.) in ischemia and reoxygenation, dimethyl sulfoxide (DMSO) was added to "trap" evolving HO. in normal, in ischemic, and in ischemic and reoxygenated rat kidney slices, incubated in short-term organ culture in vitro.
S Fukui et al.
Journal of chromatography, 630(1-2), 187-193 (1993-02-05)
For the determination of hydroxyl radicals, dimethyl sulphoxide was used as a molecular probe and the methanesulphinic acid produced was determined by high-performance liquid chromatography of its Fast Yellow GC salt derivative. The results for hydroxyl radicals formed using the
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.

![1,8-Diazabicyclo[5.4.0]undec-7-en (1,5-5) 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)