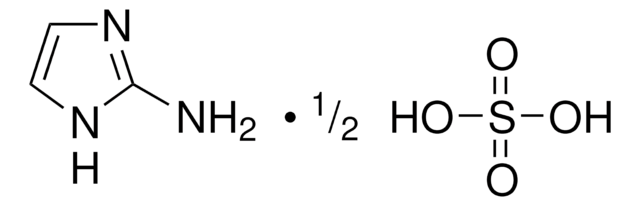Alle Fotos(1)
Wichtige Dokumente
422231
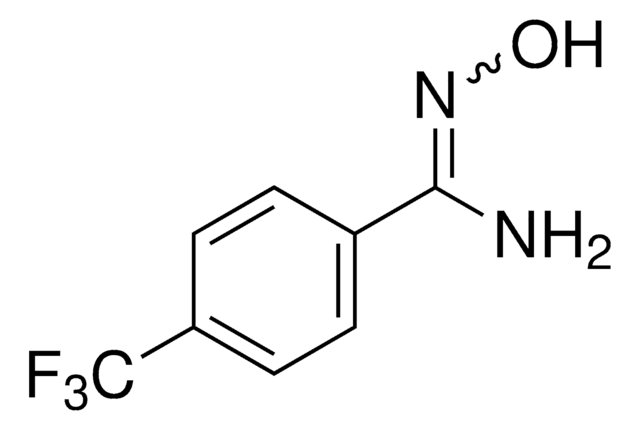
4-(Trifluormethoxy)benzamidoxim
97%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
CF3OC6H4C(=NOH)NH2
CAS-Nummer:
Molekulargewicht:
220.15
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
97%
mp (Schmelzpunkt)
113-115 °C (lit.)
Funktionelle Gruppe
amine
fluoro
oxime
SMILES String
N\C(=N/O)c1ccc(OC(F)(F)F)cc1
InChI
1S/C8H7F3N2O2/c9-8(10,11)15-6-3-1-5(2-4-6)7(12)13-14/h1-4,14H,(H2,12,13)
InChIKey
COHKFOZYLCDVRK-UHFFFAOYSA-N
Allgemeine Beschreibung
4-(Trifluoromethoxy)benzamidoxime (4-TFMBAO) is a benzamidoxime (BAO) derivative containing amidoxime functional group. Its density and freezing point have been determined.
Anwendung
4-(Trifluoromethoxy)benzamidoxime (4-TFMBAO) is suitable reactant in the fluorescence (FL) deriving reaction, one of the widely-used methodology specifically used to quantify uracil. It may be used as a reactant in the synthesis of oxadiazoles. It may also be used a fluorogenic agent in the quantification of orotic acid by spectrofluorometric method in human biological specimens.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Sensitive and Selective Determination of Orotic Acid in Biological Specimens Using a Novel Fluorogenic Reaction.
Yin S, et al.
Journal of Fluorescence, 25(4), 1005-1011 (2015)
Thomas E Barta et al.
Bioorganic & medicinal chemistry letters, 21(10), 2820-2822 (2011-04-22)
Seeking compounds preferentially potent and selective for MMP-13, we reported in the preceding Letter on a series of hydroxamic acids with a flexible benzamide tail groups.(1a) Here, we replace the amide moiety with non-hydrolyzable heterocycles in an effort to improve
Yaws CL.
The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals, 185-185 (2015)
Evan R Abt et al.
Cell chemical biology, 27(2), 197-205 (2019-11-18)
Biosynthesis of the pyrimidine nucleotide uridine monophosphate (UMP) is essential for cell proliferation and is achieved by the activity of convergent de novo and salvage metabolic pathways. Here we report the development and application of a cell-based metabolic modifier screening
Takayuki Shibata et al.
Analytica chimica acta, 674(2), 234-238 (2010-08-04)
Facile and specific methods to quantify a nucleobase in biological samples are of great importance for diagnosing disorders in nucleic acid metabolism. In the present study, a novel fluorogenic reaction specific for uracil has been developed. The reaction was carried
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.