Wichtige Dokumente
414492
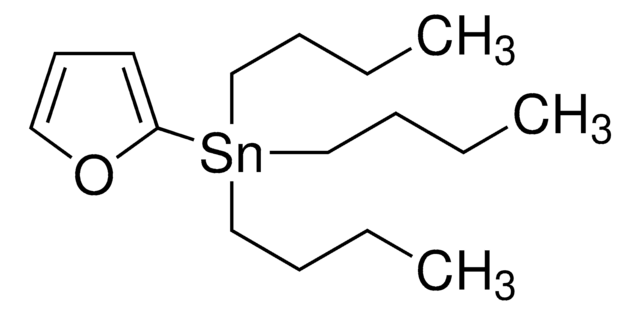
2-(Tributylstannyl)thiophen
97%
Synonym(e):
2-(Tributyltin)thiophene, 2-Thienyltributyltin, Tributyl(thiophen-2-yl)stannate, Tributyl-2-thienylstannane, Tributylthiophen-2-ylstannane
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Form
liquid
Brechungsindex
n20/D 1.518 (lit.)
bp
155 °C/0.1 mmHg (lit.)
Dichte
1.175 g/mL at 25 °C (lit.)
SMILES String
CCCC[Sn](CCCC)(CCCC)c1cccs1
InChI
1S/C4H3S.3C4H9.Sn/c1-2-4-5-3-1;3*1-3-4-2;/h1-3H;3*1,3-4H2,2H3;
InChIKey
UKTDFYOZPFNQOQ-UHFFFAOYSA-N
Anwendung
Signalwort
Danger
Gefahreneinstufungen
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2 - STOT RE 1
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flammpunkt (°F)
221.0 °F - closed cup
Flammpunkt (°C)
105 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 414492-10ML | 4061838054067 |
| 414492-250ML | 4061838127310 |
| 414492-50ML | 4061838127327 |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.

![1,1′-[4,8-Bis[5-(2-ethylhexyl)-2-thienyl]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl]bis[1,1,1-trimethylstannane]](/deepweb/assets/sigmaaldrich/product/structures/611/912/a638a6fe-ca7b-4674-8023-df4c0921a9fd/640/a638a6fe-ca7b-4674-8023-df4c0921a9fd.png)
![2,5-Bis(trimethylstannyl)-thieno[3,2-b]thiophene 97%](/deepweb/assets/sigmaaldrich/product/structures/126/532/26557e94-858e-4c96-90de-ca88d84a8727/640/26557e94-858e-4c96-90de-ca88d84a8727.png)