Alle Fotos(2)
Wichtige Dokumente
389439
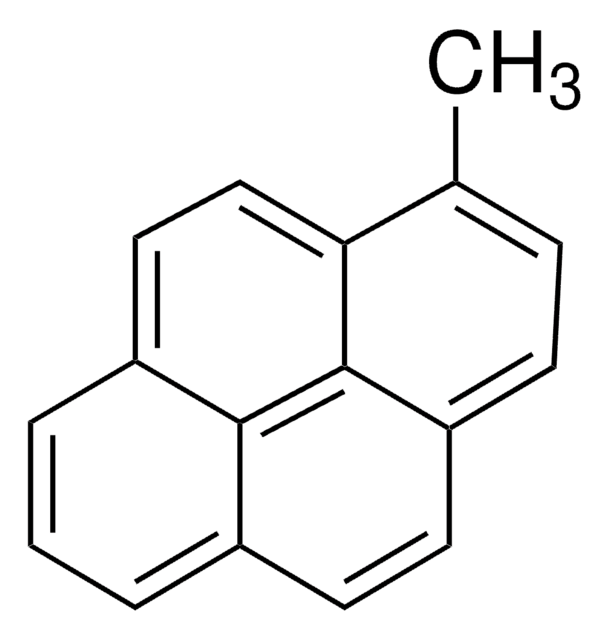
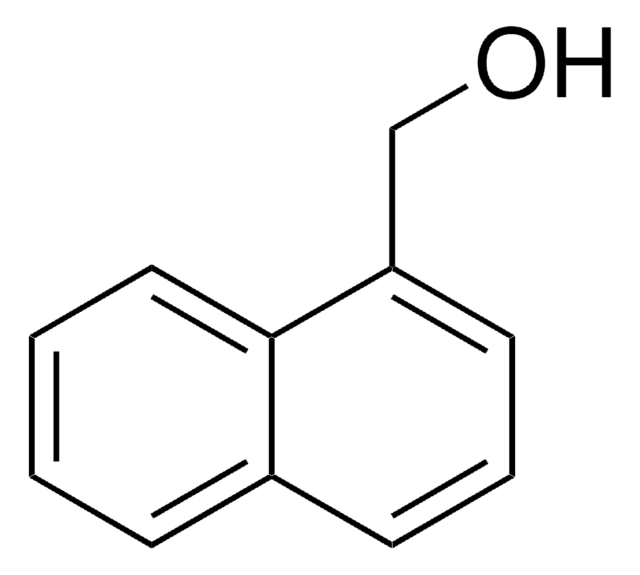
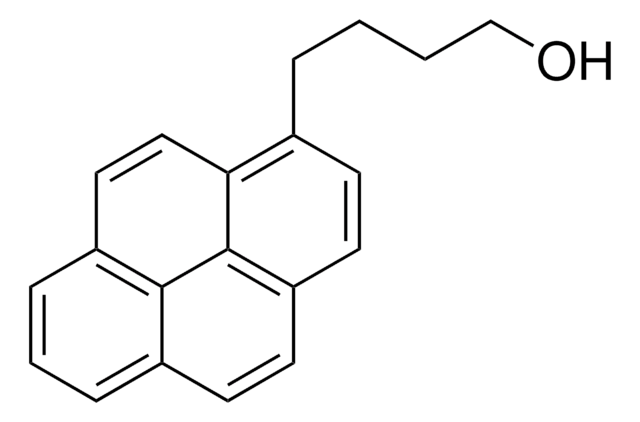
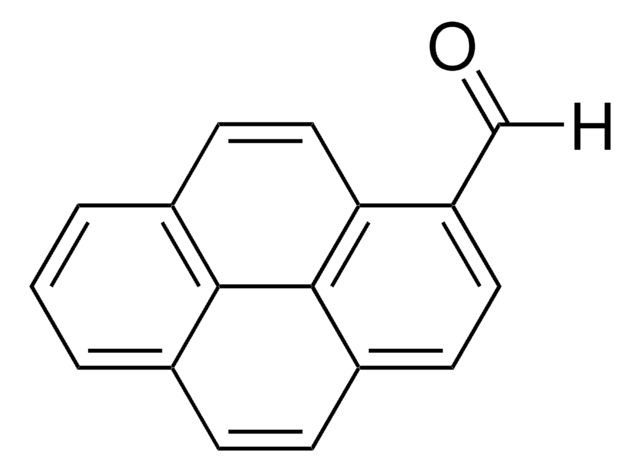
1-Pyrenmethanol
98%
Synonym(e):
1-(1-Hydroxymethyl)pyrene, 1-Hydroxymethylpyrene
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C17H12O
CAS-Nummer:
Molekulargewicht:
232.28
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Form
solid
mp (Schmelzpunkt)
123-126 °C (lit.)
Funktionelle Gruppe
hydroxyl
SMILES String
OCc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C17H12O/c18-10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)17(13)16(11)12/h1-9,18H,10H2
InChIKey
NGDMLQSGYUCLDC-UHFFFAOYSA-N
Anwendung
1-Pyrenemethanol can be used:
- For the synthesis of pincer-like benzene-bridged fluorescent selective sensor for adenosine-5′-triphosphate (ATP) detection.
- As a starting material for the synthesis of pyrene-end poly(glycidyl methacrylate) polymer.
- As an initiator for the synthesis of pyrene core star polymers.
- For the synthesis of 1-pyrenecarboxaldehyde, an important intermediate in pharmaceutical and agrochemical fields.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
H Glatt et al.
Carcinogenesis, 14(4), 599-602 (1993-04-01)
1-Hydroxymethylpyrene (HMP), a primary benzylic alcohol, and 4H-cyclopenta[def]chrysen-4-ol (OH-CPC), a secondary benzylic alcohol, were investigated for mutagenicity in Salmonella typhimurium (reversion of the his- strain TA98) in the presence of various xenobiotic-metabolizing systems. In the direct test, HMP was inactive
Encapsulation of functional moieties within branched star polymers: effect of chain length and solvent on site isolation.
Hecht S, et al.
Journal of the American Chemical Society, 123(1), 18-25 (2001)
Walter Meinl et al.
Pharmacogenetics, 12(9), 677-689 (2002-12-05)
Various enzymatically formed sulfuric acid esters are chemically reactive and mutagenic. This metabolic activation pathway is not detected in standard in-vitro mutagenicity test systems. We describe the construction of Salmonella typhimurium TA1538-derived strains expressing alloenzymes *1, *2, *3, *5, *6
Highly efficient oxidation of alcohols to carbonyl compounds in the presence of molecular oxygen using a novel heterogeneous ruthenium catalyst.
Ji H, et al.
Tetrahedron Letters, 43(40), 7179-7183 (2002)
H Glatt et al.
Chemico-biological interactions, 109(1-3), 195-219 (1998-05-05)
Sulfation is a common final step in the biotransformation of xenobiotics and is traditionally associated with inactivation. However, the sulfate group is electron-withdrawing and may be cleaved off heterolytically in some molecules leading to electrophilic cations which may form adducts
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 389439-10G | 4061831970791 |
| 389439-1G | 4061831970852 |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)