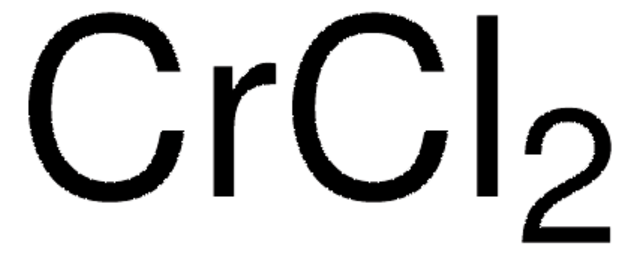Alle Fotos(1)
Wichtige Dokumente
324647
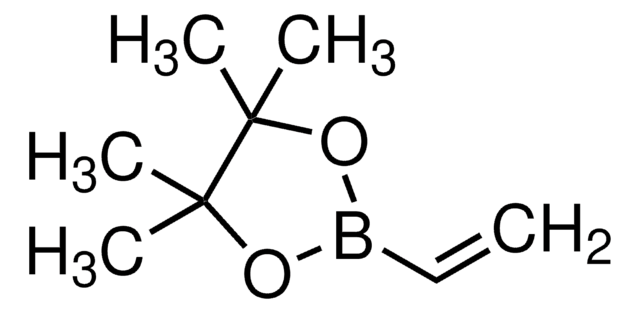
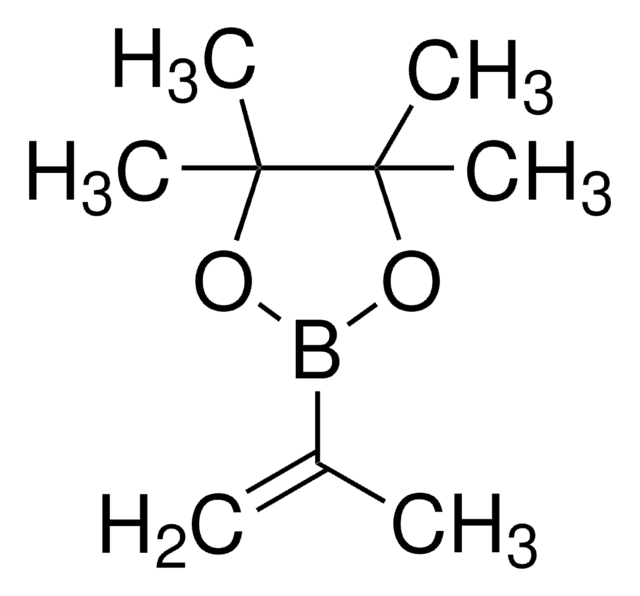
2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolan
97%
Synonym(e):
Pinacol-allylboronat
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
((CH3)4C2O2)BCH2CH=CH2
CAS-Nummer:
Molekulargewicht:
168.04
MDL-Nummer:
UNSPSC-Code:
12352103
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Brechungsindex
n20/D 1.4268 (lit.)
bp
50-53 °C/5 mmHg (lit.)
Dichte
0.896 g/mL at 25 °C (lit.)
Lagertemp.
2-8°C
SMILES String
CC1(C)OB(CC=C)OC1(C)C
InChI
1S/C9H17BO2/c1-6-7-10-11-8(2,3)9(4,5)12-10/h6H,1,7H2,2-5H3
InChIKey
YMHIEPNFCBNQQU-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
Allylboronic acid pinacol ester is used as a nucleophile in the catalytic allylation of simple ketoimines.
Anwendung
Reagent used for
Reagent used in Preparation of
- Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions and olefin metathesis
- Intermolecular radical additions
- Allylboration of aldehydes catalyzed by chiral spirobiindane diol (SPINOL) based phosphoric acids
- Cobalt-catalyzed regioselective hydrovinylation of dienes with alkenes
- Nucleic acid-templated energy transfer leading to a photorelease reaction
- Stereoselective indium-catalyzed Hosomi-Sakurai reactions
Reagent used in Preparation of
- Cyclic sulfone hydroxyethylamines as BACE1 inhibitors for reduction of Amyloid β-Peptides
- Transmetalation of carbene Ru iodide with Ag carboxylates to give C-H-activated Ru carbene complexes as catalysts for Z-selective olefin metathesis
- Allylboronation reagent for the preparation of allylic alcohols, and homoallylic amines.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Flam. Liq. 3
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 3
Flammpunkt (°F)
114.8 °F - closed cup
Flammpunkt (°C)
46 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Manuel Röthlingshöfer et al.
Journal of the American Chemical Society, 133(45), 18110-18113 (2011-10-19)
The photocleavage of a nitrobenzyl-type linker (NPPOC) at 405 nm wavelength was enabled by nucleic acid-templated energy transfer from a sensitizer (thioxanthenone) to the linker. This strategy was used to release profluorescent rhodamine, which facilitated monitoring of the reaction via
A catalytic asymmetric borono variant of Hosomi-Sakurai reactions with N,O-aminals.
Yi-Yong Huang et al.
Angewandte Chemie (International ed. in English), 50(47), 11121-11124 (2011-10-07)
Heinrich Rueeger et al.
Journal of medicinal chemistry, 55(7), 3364-3386 (2012-03-03)
Structure-based design of a series of cyclic hydroxyethylamine BACE1 inhibitors allowed the rational incorporation of prime- and nonprime-side fragments to a central core template without any amide functionality. The core scaffold selection and the structure-activity relationship development were supported by
Gaining absolute control of the regiochemistry in the cobalt-catalyzed 1,4-hydrovinylation reaction.
Marion Arndt et al.
Organic letters, 13(23), 6236-6239 (2011-11-02)
The absolute control of the regiochemistry of a cobalt-catalyzed 1,4-hydrovinylation reaction is achieved by alternation of the ligands applied. While the dppe/dppp ligands led to the formation of the branched product, the herein described application of the SchmalzPhos ligand generates
Organometallics, 2, 230-230 (1983)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.