Alle Fotos(1)
Wichtige Dokumente
246298
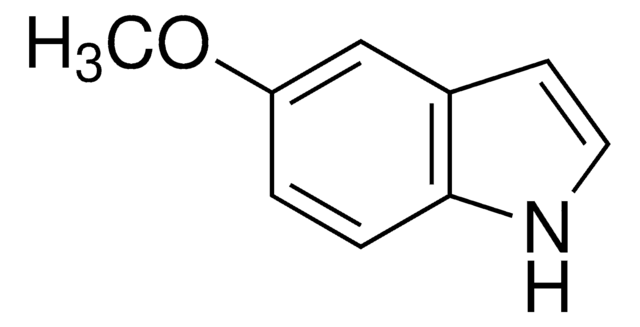
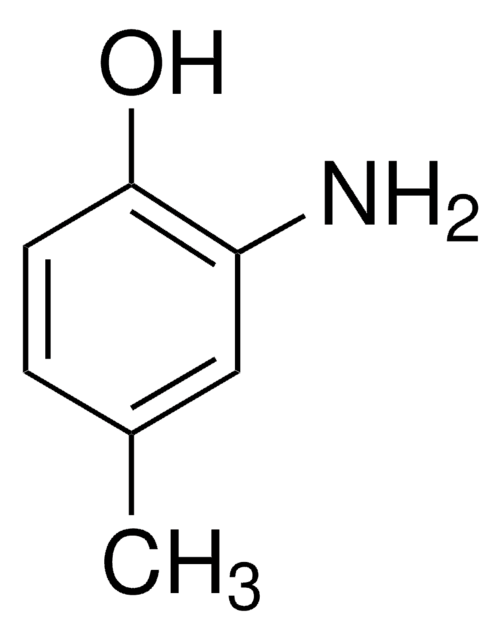
4-Methoxyindol
99%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C9H9NO
CAS-Nummer:
Molekulargewicht:
147.17
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
99%
Form
powder
bp
181-183 °C/24 mmHg (lit.)
mp (Schmelzpunkt)
69-70 °C (lit.)
Löslichkeit
ethanol: 50 mg/mL, clear, faintly yellow
SMILES String
COc1cccc2[nH]ccc12
InChI
1S/C9H9NO/c1-11-9-4-2-3-8-7(9)5-6-10-8/h2-6,10H,1H3
InChIKey
LUNOXNMCFPFPMO-UHFFFAOYSA-N
Verwandte Kategorien
Anwendung
4-Methoxyindole was used for comparing the complexation reaction of β-cyclodextrin (β-CD) with pindolol using reversed-phase liquid chromatography.
Reactant for preparation of:
- GABA analogs
- Sodium-Dependent Glucose Co-transporter 2 (SGLT2) Inhibitors for the Management of Hyperglycemia in Diabetes
- Anticancer agents
- Integrase strand-transfer inhibitors (INSTIs)
- Inhibitor of Proliferation of Colon Cancer Cells
- Isomeridianin G as GSK-3ß inhibitors
- HIV-1 integrase inhibitors
- Inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2)
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Carmen Gazpio et al.
Journal of pharmaceutical and biomedical analysis, 37(3), 487-492 (2005-03-03)
The complexation with beta-cyclodextrin (beta-CD) has been investigated using reversed-phase liquid chromatography. The compounds tested have been pindolol and, for comparison purposes, indole and 4-methoxyindole. The retention behaviour has been analysed on a Kromasil 100 C18 column and the mobile
Brenden Barco et al.
Nature communications, 10(1), 3444-3444 (2019-08-03)
Plants synthesize numerous ecologically specialized, lineage-specific metabolites through biosynthetic gene duplication and functional specialization. However, it remains unclear how duplicated genes are wired into existing regulatory networks. We show that the duplicated gene CYP82C2 has been recruited into the WRKY33 regulon
Thomas Heine et al.
Applied biochemistry and biotechnology, 181(4), 1590-1610 (2016-11-11)
The enantioselective epoxidation of styrene and related compounds by two-component styrene monooxygenases (SMOs) has targeted these enzymes for development as biocatalysts. In the present work, we prepare genetically engineered fusion proteins that join the C-terminus of the epoxidase (StyA) to
Jung Min Song et al.
International journal of pharmaceutics, 477(1-2), 96-101 (2014-10-15)
Indole-3-carbinol (I3C), a constituent of commonly consumed Brassica vegetables, has been shown to have anticancer effects in a variety of preclinical models of lung cancer. However, it has shown only limited efficacy in clinical trials, likely due to its poor
Tien-Yuan Wu et al.
Journal of pharmacokinetics and pharmacodynamics, 42(4), 401-408 (2015-07-04)
3,3'-Diindolylmethane (DIM) has been investigated as a potential anti-cancer chemopreventive agent in many preclinical and clinical studies. In this study, we sought to characterize the pharmacokinetics of DIM and to build a pharmacokinetic (PK) and pharmacodynamic (PD) model of the
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.