22110
(+)-Catechin Hydrat
≥96.0% (sum of enantiomers, HPLC)
Synonym(e):
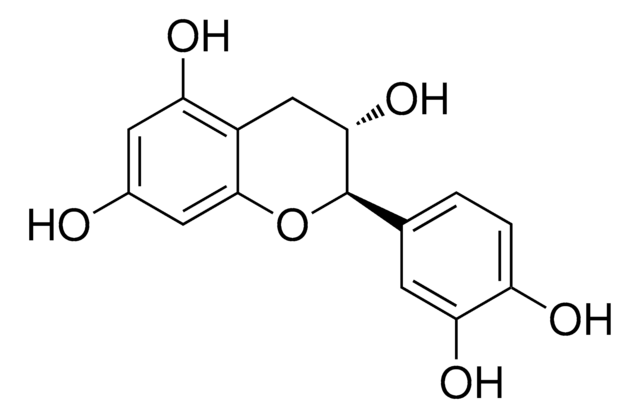
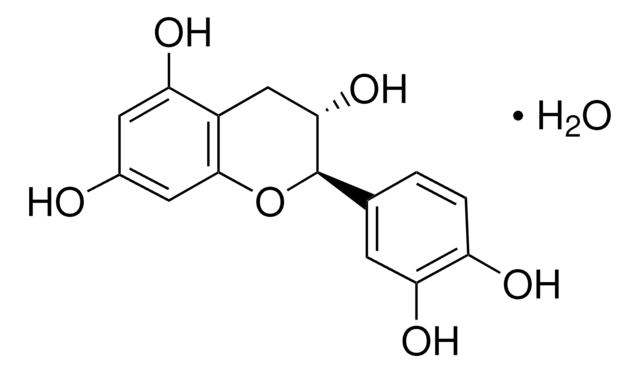
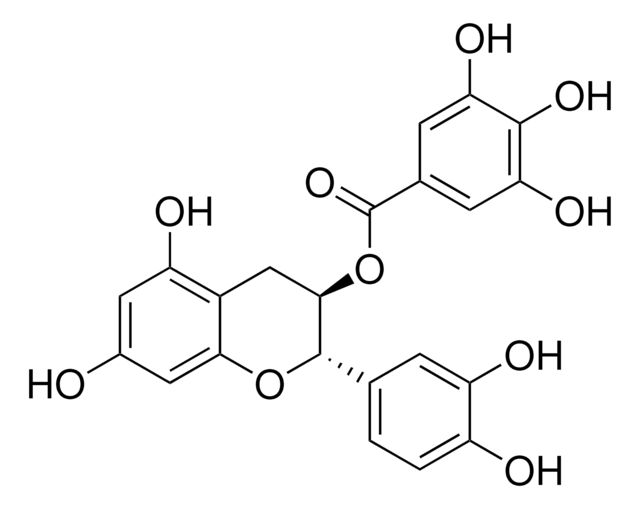
(+)-Cyanidol-3, (2R,3S)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol
About This Item
Empfohlene Produkte
Assay
≥96.0% (sum of enantiomers, HPLC)
Form
powder
Optische Aktivität
[α]/D +26±2°, c = 1 in H2O
Verunreinigungen
≤8% water
mp (Schmelzpunkt)
175-177 °C (anhydrous) (lit.)
Lagertemp.
2-8°C
SMILES String
[H]O[H].O[C@H]1Cc2c(O)cc(O)cc2O[C@@H]1c3ccc(O)c(O)c3
InChI
1S/C15H14O6.H2O/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7;/h1-5,13,15-20H,6H2;1H2/t13-,15+;/m0./s1
InChIKey
OFUMQWOJBVNKLR-NQQJLSKUSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- As an inhibitor of steel corrosion in hydrochloric acid solution.
- As a model compound in the study of antimicrobial activities of flavonoids on Escherichia coli.
- As a starting material for the synthesis of catechin glucosides of biological importance.
Vorsicht
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Coumaric acid; Quercitrin; Myricetin; Quercetin
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Protokolle
Coumaric acid; Quercitrin; Myricetin; Quercetin
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.